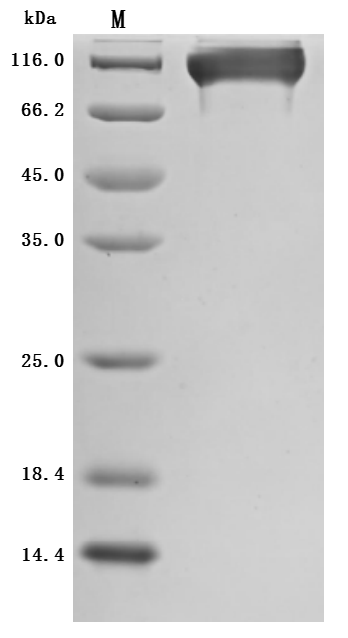
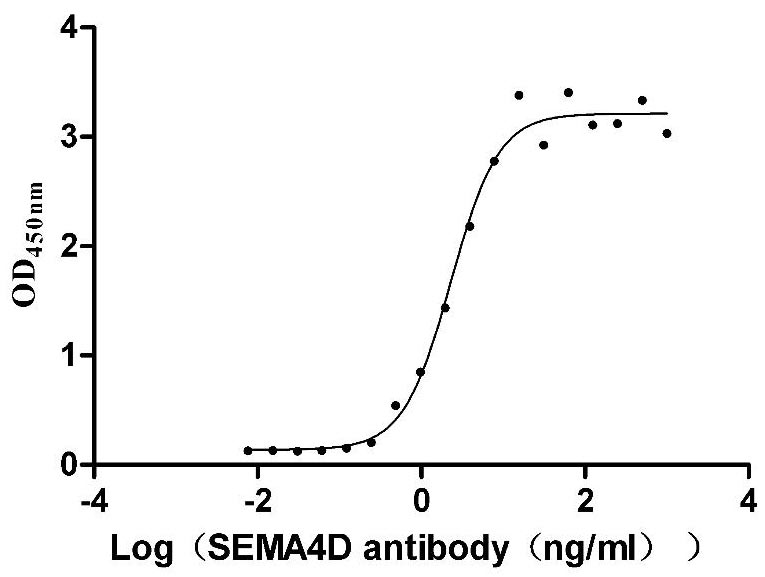
This recombinant Macaca mulatta SEMA4D protein (amino acids 24-734) is produced in mammalian cells with C-terminal 10×His tagging. The preparation demonstrates excellent purity (>95% by SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method), making it suitable for immunological and neurological research applications. Functional characterization reveals high-affinity binding to anti-SEMA4D antibody (CSB-RA835707A1HU) in ELISA (EC50: 1.943-2.724 ng/mL at 2 μg/mL immobilization), confirming its structural integrity. The mammalian expression system ensures proper glycosylation and folding, which are critical for SEMA4D's biological activity in axon guidance and immune modulation. Presented as lyophilized powder, this protein offers stability and convenient handling. The C-terminal tag facilitates purification without disrupting functional domains. This recombinant SEMA4D protein serves as a valuable tool for studying semaphorin-mediated signaling in neural development, immune responses, and tumor microenvironment interactions.
The SEMA4D protein, encoded by the SEMA4D gene, is a member of the semaphorin family, which are crucial modulators of neural and immune system functions. In Macaca mulatta, commonly known as the rhesus macaque, the study of SEMA4D has provided insights into a variety of biological processes and disease models, particularly concerning immune responses and its implications for understanding human diseases, such as HIV infections.
SEMA4D serves as a significant signal in the immune system, influencing T-cell behavior and dendritic cell functions. Research indicates that SEMA4D is expressed in various immune cells, and its interaction with the receptor plexin-B1 regulates the immune response by affecting dendritic cell function and T-cell activation, thereby also contributing to the modulation of inflammation and immune tolerance [1]. The protein's role appears particularly critical in the context of viral infections, including HIV, where it may influence the course of infection and immune response in the host, making it a potential target for therapeutic interventions [2].
Moreover, Macaca mulatta serves as an invaluable model due to its close genetic and physiological proximity to humans. This is manifest in the study of SEMA4D in the context of simian immunodeficiency virus (SIV) and other diseases, highlighting the relevance of this protein in translational research [1]. The SEMA4D protein's mechanisms might not only illuminate the pathophysiology of the disease but also inform future therapeutic strategies in human immunology [3].
In addition to immunological studies, SEMA4D's potential involvement in cancer biology has garnered interest. Research indicates that disruptions in semaphorin signaling, including that mediated by SEMA4D, can contribute to tumor biology and immune evasion, parallel to findings in human cancers [2]. The protein's interactions suggest regulatory roles that could be exploited for therapeutic advancements in oncology and vaccine development [2].
In summary, the SEMA4D protein in Macaca mulatta represents a key element in understanding complex interactions in the immune system, particularly concerning viral infections and cancer biology, highlighting the utility of this primate model in advancing medical research.
References:
[1] S. Handley, C. Desai, et al. Siv infection-mediated changes in gastrointestinal bacterial microbiome and virome are associated with immunodeficiency and prevented by vaccination. Cell Host & Microbe, vol. 19, no. 3, p. 323-335, 2016. https://doi.org/10.1016/j.chom.2016.02.010
[2] B. Bimber, R. Ramakrishnan, et al. Whole genome sequencing predicts novel human disease models in rhesus macaques. Genomics, vol. 109, no. 3-4, p. 214-220, 2017. https://doi.org/10.1016/j.ygeno.2017.04.001
[3] D. An, R. Donahue, et al. Stable reduction of ccr5 by rnai through hematopoietic stem cell transplant in non-human primates. Proceedings of the National Academy of Sciences, vol. 104, no. 32, p. 13110-13115, 2007. https://doi.org/10.1073/pnas.0705474104