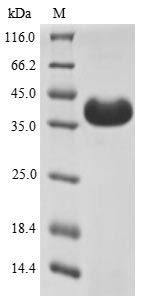
Recombinant Bovine Odorant-binding protein gets expressed in a yeast system and comes with an N-terminal 6xHis-sumostar tag that makes purification and detection much simpler. The complete protein covers amino acids 1 to 159. Purity appears to exceed 90% based on SDS-PAGE analysis. This product is meant for research use only and works well for different biochemical and structural studies that need high-purity protein.
Odorant-binding proteins (OBPs) are small, soluble proteins that transport odor molecules around. They seem to play a key role in how we detect and tell apart different smells by grabbing onto odorant molecules and helping move them to olfactory receptors. For researchers, OBPs matter because they help us figure out how smell works. Scientists can use them in studies about sensory biology and how molecules recognize each other.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant bovine odorant-binding protein (OBP) is unlikely to be correctly folded or fully bioactive for its native functions without further validation. OBP is a small soluble protein that requires precise tertiary structure to form a hydrophobic binding pocket for odorant molecules. The N-terminal 6xHis-SUMOstar tag (approximately 15-20 kDa) is substantially larger than the OBP domain (∼18 kDa) and may sterically hinder proper folding, particularly if the odorant-binding site is near the N-terminus. While the yeast expression system provides a eukaryotic environment that could support disulfide bond formation and solubility, the large fusion tag likely disrupts the native conformation. The purity >90% indicates low contaminants, but does not guarantee correct folding of the OBP domain. Without experimental validation (e.g., circular dichroism for secondary structure, fluorescence quenching assays for ligand binding), the protein's bioactivity remains uncertain.
This recombinant OBP is suitable for antibody generation, but antibodies will primarily target the immunodominant SUMOstar tag rather than OBP-specific epitopes. For antibodies recognizing native OBP conformations, the tag should be removed before immunization. As a standard in immunoassays, the tagged protein can work, but cross-reactivity with the tag must be controlled via tag-specific controls. Consistency is high, but specificity for OBP may be low.
To ensure reliable outcomes, first remove the SUMOstar tag using SUMO protease (if the construct includes a cleavage site) and purify the tag-free OBP; then validate folding using circular dichroism to confirm expected beta-rich secondary structure and fluorescence-based binding assays with known odorants (e.g., aldehydes or alcohols) to confirm bioactivity. If tag removal is not feasible, limit applications to tag-centric uses like anti-SUMOstar antibody development, and avoid quantitative functional studies. For structural work, always use tag-free protein and confirm homogeneity via size-exclusion chromatography. Incorporate controls for tag effects in all experiments to avoid misinterpretation.