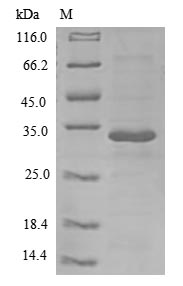
Recombinant Human Interleukin-26 (IL26) is expressed in E. coli and contains the full length of the mature protein from amino acids 22 to 171. The protein includes an N-terminal 6xHis-B2M tag, which makes purification and detection more straightforward. Analysis by SDS-PAGE confirms purity greater than 85%, which should be adequate for most research applications requiring high-quality protein.
Interleukin-26 (IL26) is a cytokine that appears to be involved in immune response regulation. It seems to play a role in modulating inflammation and immune signaling, though its precise mechanisms may still be incompletely understood. IL26 belongs to the IL-10 cytokine family and is thought to influence pathways related to immune cell communication. Studying this protein could be valuable for understanding immune system dynamics and how inflammatory processes are regulated in both health and disease states.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant human IL26 is expressed in E. coli, a prokaryotic system that is generally unsuitable for producing functional eukaryotic cytokines like IL26. IL26 is a complex alpha-helical cytokine that requires precise folding, proper disulfide bond formation, and homodimerization for its biological activity. While the protein is expressed as the mature form (22-171aa) with an N-terminal 6xHis-B2M tag and >85% purity, E. coli lacks the eukaryotic chaperones, disulfide isomerases, and quality control machinery necessary for correct folding of complex cytokines. The large B2M fusion tag (β-2-microglobulin, ~12 kDa) may significantly alter the protein's structure and interfere with its receptor-binding domains. Since activity is unverified, the protein cannot be assumed to be correctly folded or bioactive without experimental validation of its cytokine activity and receptor binding capability.
1. Antibody Development and Validation Studies
The recombinant IL26 can serve as an effective immunogen for generating antibodies that recognize linear epitopes, even if the protein is misfolded. The fusion tag facilitates purification and detection. However, antibodies may not recognize conformational or quaternary structure-dependent epitopes of native, properly folded, and dimerized IL26. Validation against IL26 from mammalian expression systems is essential.
2. Protein-Protein Interaction Studies
This application is high-risk without proper folding validation. While the His-tag enables technical feasibility for pull-down assays, if IL26 is misfolded (as likely in E. coli with a large fusion tag), it will not interact physiologically with true receptors (IL-10R2/IL-20R1). Cytokine-receptor interactions require precise three-dimensional conformation. This application should not be pursued without confirmation of proper folding and receptor binding activity.
3. Biochemical Characterization and Stability Studies
This application is well-suited for assessing the recombinant human IL26 itself. Techniques like size-exclusion chromatography with multi-angle light scattering, circular dichroism spectroscopy, and thermal shift assays can evaluate the protein's folding state, oligomerization, and stability. These studies are valuable for quality control, even if the protein is inactive.
4. Cell-Based Binding and Uptake Studies
This application is highly problematic without activity verification. If IL26 is misfolded, cell-based studies will yield misleading results. The protein requires proper dimerization and receptor-binding interfaces for physiological cellular responses. This application requires prior demonstration of proper folding and validation with known IL26-responsive cell systems.
Final Recommendation & Action Plan
Given the high probability of misfolding in E. coli for this complex cytokine with a large fusion tag, recommend first performing comprehensive validation: 1) Biophysical characterization (analytical ultracentrifugation for oligomeric state, circular dichroism for secondary structure) to assess folding quality; 2) Functional validation using IL26-responsive cell assays (STAT activation, cytokine production); 3) If possible, comparison with IL26 from mammalian expression systems. Antibody development can proceed as the safest application. Avoid all functional studies (interactions, cell-based assays) until proper folding and bioactivity are confirmed. For reliable IL26 research, obtain the protein from mammalian expression systems without large fusion tags. Always include appropriate controls, such as known active IL26 standards, in experiments.