Interferons are named for their ability to "interfere" with viral replication by protecting cells from virus infections. As an important class of CUSABIO cytokine proteins collection, we've got some questions from researchers doing the related research. Combining their questions and our exploration, we write this article.
1. What is Interferon
Interferon, also known as IFN, is a kind of cytokines which plays important roles in viral defense, tumor inhibition and disease treatment through cytotoxic T cells, NK cells and DC cells, etc. Interferon, produced mainly by monocytes and macrophages and positioned at different cells'surface, regulates cell growth, cell differentiation, immunoediting. CUSABIO has produced various interferon related products, including proteins, antibodies and Elisa kits.
(sourcing: https://en.wikipedia.org/wiki/Interferon)
2. Classification of Interferon
The IFN family is consist of three main classes of cytokines, type I IFNs, type II IFN and type III IFN. Among of them, type I IFN and type II IFN are well-studied.
Type I interferon
Type I interferon includes interferon α(which can be further divided into 13 different subtypes(IFN-α1, -α2, -α4, -α5, -α6, -α7, -α8, -α10, -α13, -α14, -α16, -α17 and -α21), β, ε, к, and ω in human. All type I IFNs bind a identical common cell-surface receptor, which is known as the type I IFN receptor. The receptor for type I interferons has multichain structures, which is composed of at least two different submits, IFNAR1 and IFNAR2. Amounting research results demonstrate that type I IFNs plays a key role in microbial infection. However, each member of type I IFNs also has its unique biologic functions. For the instance, although both IFN-α and IFN-β are elicited by Cl-13 infection, losing of IFN-β signaling is the primary factor that initiates early clearance of LCMV persistent infection by blocking IFNAR. in the stage of early viral clearance, the result of blocking IFN-β similar to that observed by blocking the IFNAR despite the presence of high levels of IFN-α; The study of Ng, C.T et al. reports that blockade of at least six forms of IFN-α can not accelerate viral clearance[1]. However, IFN-α was key to controlling viral spread, as only IFN-α blockade altered early viral dissemination, manifesting that the roles played by IFN-β as compared to IFN-α in controlling viral infection are different[2].
Type II Interferon
Type II interferon, only including interferon gamma, also known as IFN γ, is a cytokine that is critical for innate and adaptive immunity against viral, some protozoal and bacterial infections. Comparing with type I interferon, the protein does not exist marked structural homology. The receptor for type II interferons also has multichain structures, which is composed of two different submits, IFNGR1 and IFNGR2. IFN γ is Produced by lymphocytes, activated by specific antigens or mitogens, and has an antiviral activity and important immunoregulatory functions. It is a potent activator of macrophages, and has antiproliferative effects on transformed cells. IFN γ is an important mediator of immunity and inflammation that utilizes the JAK-STAT signaling pathway to activate the STAT1 transcription factor. In addition, emerging evidence shows that it can enforce the antiviral and antitumor effects of the type I interferons.
Type III Interferon
Type III interferon includes interferon λ, which can be further divided into 4 distinct subtypes, IFNλ1, IFNλ2, IFNλ3 and IFNλ4 in human. However, IFNλ1-λ4 are pseudogenes in mice, which prevents the function study of these cytokines in this animal model [3][4].
The members of interferon family are shown in the table 1.
Table1 The member of IFNs family
|
Gene members
|
Uniprot ID
|
Protein Name
|
Receptor&Description
|
|
IFNα1
|
P01562
|
Interferon alpha-1
|
IFNAR1: Interferon alpha/beta receptor 1 is a component of the receptor for type I interferons. Its function in general as heterodimer with IFNAR2. Type I interferon binding activates the JAK-STAT signaling cascade, and triggers tyrosine phosphorylation of a number of proteins including JAKs, TYK2, STAT proteins and the IFNR alpha- and beta-subunits themselves. Can form an active IFNB1 receptor by itself and activate a signaling cascade that does not involve activation of the JAK-STAT pathway.
IFNAR2: Interferon alpha/beta receptor 2, associated with IFNAR1 to form the type I interferon receptor, is receptor for interferons alpha and beta. It is Involved in IFN-mediated STAT1, STAT2 and STAT3 activation. Isoform 1 and isoform 2 are directly involved in signal transduction due to their association with the TYR kinase, JAK1. Isoform 3 is a potent inhibitor of type I IFN receptor activity.
|
|
IFNα2
|
P01563
|
Interferon alpha-2
|
|
IFNα4
|
P05014
|
Interferon alpha-4
|
|
IFNα5
|
P01569
|
Interferon alpha-5
|
|
IFNα6
|
P05013
|
Interferon alpha-6
|
|
IFNα7
|
P01567
|
Interferon alpha-7
|
|
IFNα8
|
P32881
|
Interferon alpha-8
|
|
IFNα10
|
P01566
|
Interferon alpha-10
|
|
IFNα13
|
P01562
|
Interferons alpha-13
|
|
IFNα14
|
P01570
|
Interferon alpha-14
|
|
IFNα16
|
P05015
|
Interferon alpha-16
|
|
IFNα17
|
P01571
|
Interferon alpha-17
|
|
IFNα21
|
P01568
|
Interferon alpha-21
|
|
IFNβ
|
P01574
|
Interferon beta
|
|
IFNε
|
Q86WN2
|
Interferon epsilon
|
|
IFNκ
|
Q9P0W0
|
Interferon kappa
|
|
IFNω
|
P05000
|
Interferon omega
|
|
IFNγ
|
P01579
|
Interferon gamma
|
IFNGR1: Interferon gamma receptor 1 associates with IFNGR2 to form a receptor for the cytokine interferon gamma.
IFNGR2: Interferon gamma receptor 2 associates with IFNGR1 to form a receptor for the cytokine interferon gamma. Ligand binding stimulates activation of the JAK/STAT signaling pathway.
|
|
IFNλ1
|
Q8IU54
|
Interferon lambda-1
|
IFNLR1: Interferon lambda receptor 1 is also known as IFNLR1. The IFNLR1/IL10RB dimer is a receptor for the cytokine ligands IFNL2 and IFNL3 and mediates their antiviral activity.
IL10RB: Interleukin-10 receptor subunit beta is shared cell surface receptor required for the activation of five class 2 cytokines: IL10, IL22, IL26, IL28, and IFNL1. The IFNLR1/IL10RB dimer is a receptor for the cytokine ligands IFNL2 and IFNL3 and mediates their antiviral activity.
|
|
IFNλ2
|
Q8IZJ0
|
Interferon lambda-2
|
|
IFNλ3
|
Q8IZI9
|
Interferon lambda-3
|
|
IFNλ4
|
K9M1U5
|
Interferon lambda-4
|
3. The Interferon Signaling Pathway
In the past two decades, accumulating evidences have revealed the mechanism of the interferon signaling pathway. Since the original discovery of the classical JAK-STAT signaling pathway, it has become clear that the connection and cooperation of multiple distinct signalling cascades are required for the generation of responses to interferons, including the mitogen-activated protein kinase p38 cascade and the phosphatidylinositol 3-kinase cascade. In this part, we focus on the most fundamental classical signaling pathway[5]. According to different type of interferon and corresponding receptors, we introduce the relevant signaling pathway, respectively.
Type I IFN Signaling Pathway
Almost all cell types particularly plasmacytoid dendritric cells(pDC) upon virus recognition can produce type I interferon. The receptors of type I interferon make up of IFNAR1 and IFNAR2. They stimulate the JAK-STAT pathway, resulting in the expression of IFN-stimulated genes(ISG), which are related to the antiviral host defense. An important transcriptional complex, induced by type I IFNs, is the ISG factor 3 (ISGF3) complex. The mature ISGF3 complex is consist of the phosphorylated STAT1 and STAT2, and combine with IRF9, which does not suffer tyrosine phosphorylation. This complex is the only complex that binds specific elements (known as IFN-stimulated response elements(ISREs)) that are present in the promoters of certain ISGs, then initiating their transcription. Overall, IFNα can be used to treat hepatitis B and C infections, while IFNβ can be used to treat multiple sclerosis. The receptors of type III interferon differ from the former one, but they have the common signaling pathway. Type III interferon plays critical roles in the antiviral host defense, predominantly in the epithelial tissues.
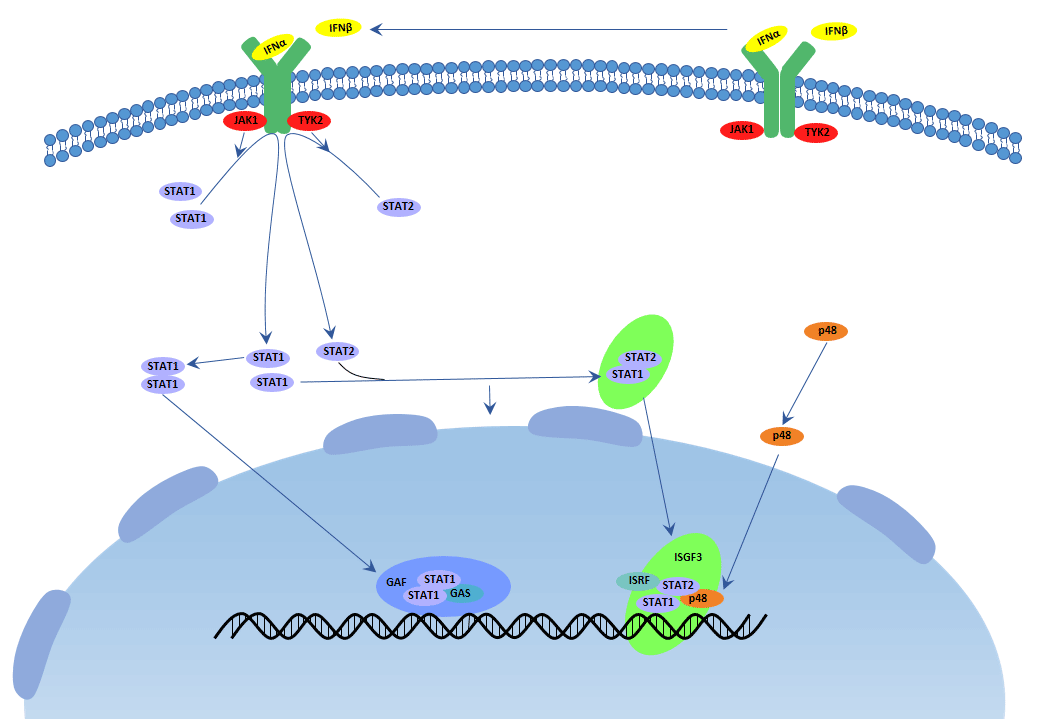
Figure 1 Type I IFN Signaling Pathway
Type II IFN Signaling Pathway
Type II interferon(interferon gamma) is produced by activating T cells, natural killer cells and macrophages et al. upon the stimuli of cytokines like interleukin 12. The receptors of type II interferon make up of IFNGR1 and IFNGR2. The transcription of type II IFN (IFN γ)-dependent genes is regulated by GAS elements, and STAT1 is the most important IFN γ-activated transcription factor for the regulation of these transcriptional responses. After binding to the type II IFN receptor by IFN γ, JAK1 and JAK2 are activated and then phosphorylate STAT1 on the tyrosine residue. Then phosphorylated STAT1 combines with another and forms STAT1–STAT1 homodimers , which translocate to the nucleus and bind GAS elements to initiate transcription. Comparing with type I IFNs, IFN γ does not induce to form ISGF3 complexes, and thereby cannot induce the transcription of genes which have only ISREs in their promoter. In addition to having antiviral activity, type II interferon has important immunoregulatory functions as it is a potent activator of macrophages and T helper 1 cells, at the same time, it has antiproliferative, antiviral and antitumor effects.
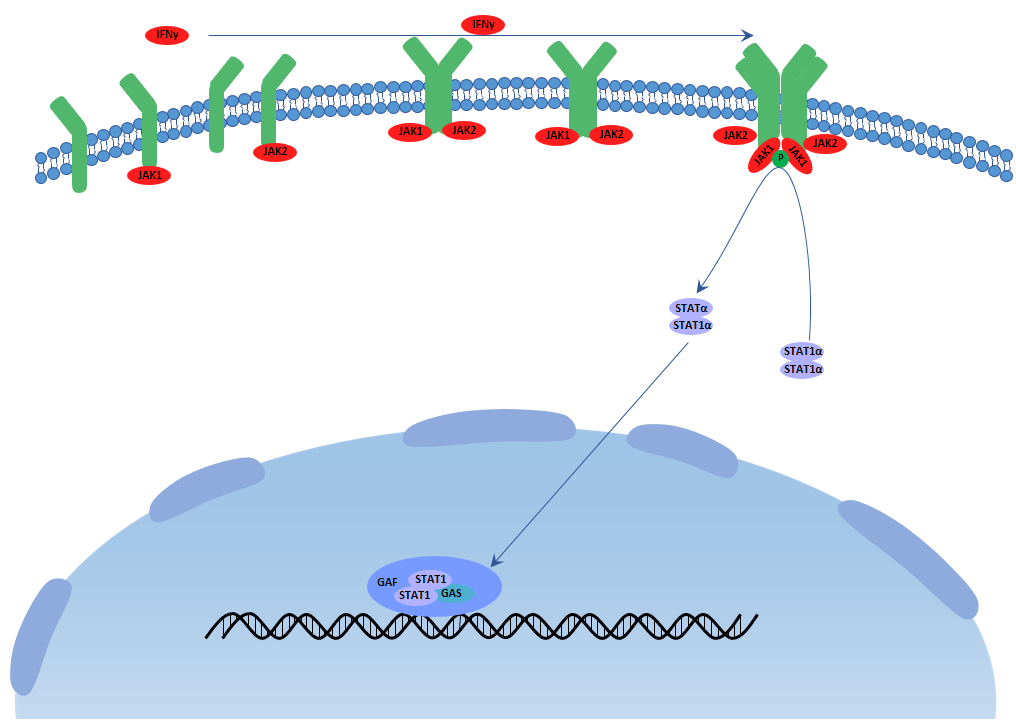
Figure 2 Type II IFN Signaling Pathway
4. The Interferon and Diseases
Interferon is so important that it is related to many diseases and the application of it should be highlighted.
Interferon gamma and Tumor
The phases of tumor immunoediting are tumor elimination, tumor dormancy and tumor escape. IFNγ takes part in every phase and the pro-infammation. IFNγ helps the induction to the apoptosis or relapse and progression of tumor.
IFNγ can induce apoptosis and nonapoptosis directly and indirectly. IFNγ can activate the expression of IRF1, a tumor suppressor, in turn, it activates the expression of Bak and reduces the expression of BCL2. These materials stimulate cytochrome c and activate caspase leading to apoptosis of tumor cells. IFNγ simulates amounts of chemicals like ROS and RNI tending to undergo apoptosis. IFNγ can also induce autophagy in human cells. IFNγ can enhance p53-mediated apoptosis and TRAIL or FAS-mediated apoptosis by STAT signaling pathway.
IFNγ can induce dormancy leading to the arrest of cancer growth depend on STAT signaling pathway directly or indirectly. It is manifested that some breast cancer genes like HER2 are dormant in some patients for a long time.
IFNγ facilitates tumor progression and relapse due to its inflammatory characteristic. Melanomas associates with macrophages which produce IFNγ. In turn, inhibiting IFNγ by UV exposure can abolish melanomas. High concentration of IFNγ may lead to NAFLD, subsequent NASH and HCC[6].
Interferon alpha and HIV
IFNα family has 13 members positioned on chromosome 9 in human. Different subtypes have high similarity in their sequence length identity as they have the same ancestor. While the affinity with their receptor, expression level and downstream signaling cascade are different from each subtype in different microenvironment. It is widely acknowledged that IFN has antiviral function. When it is induced by virus, the JAK-STAT signaling pathway is activated, the ISGs(IFN-stimulated genes) are upregulated to resist virus. However, some virus can evade from the IFN immunoregulation and the mechanism is not very clear, it is valuable to explore it deeply for the therapy.
So far, all reports on HIV or SIV infection showed different results in IFNα subtype gene expression, which strongly depended on the analyzed type of tissue, the cell or stimulus. Different IFNα subtypes induce different ISG expression patterns. Some IFNα subtypes(IFNα1, IFNα2, IFNα6, IFNα14, IFNα17 and IFNα21) strongly enhanced the mRNA expression of HIV restriction factors(Mx2, SAMHD1, Tetherin and Trim22), whereas all other IFNα subtypes only slightly increased the ISG expression compared to untreated controls in vitro. They observed that some IFNα subtypes(IFNα14, IFNα6, IFNα17 and IFNα21) potently decreased viral replication shown by cellular p24 levels and infectivity of cell culture supernatants. Interestingly, the clinically used subtype IFNα2 (for HBV therapy) only modestly suppressed HIV replication in vivo. What’s more, structural improvements of IFN might be a considerable option for future immunotherapy regimens in viral infections. It is well established that type I interferon efficiently induces antiviral restriction factors. Accumulating evidence suggests that other types of IFN, specific cytokines and other activators of the cell are also able to upregulate the expression of restriction factors and hence to establish an antiviral cellular state[7].
It was shown that the typical response to IFNα therapy for HCV-infected patients characterized by two phases of viral loads: a rapid decrease due to antiviral ISG expression and a slower decrease mediated by immune cells. Thus, the immunoregulatory activity of IFN may be required for a successful therapy of a chronic virus infection. However, type I interferon inducing immune activation can be either beneficial or harmful in HIV infection as IFNγ rather than IFNα mediates the persistent LCVM disease[2].
Interferon gamma and Tuberculosis
Tuberculosis is a serious infectious disease in our world. The defense depends on the cellular immune response mediated by T cells. IFNγ is produced by T cells, NK cells and macrophages with antiproliferation of transformed cell, antitumor and potentiating the antiviral effects of type I interferon. We obverved that mice with gko deficient could not produce IFNγ and survive well without the pathogen. After the infection of tuberculosis, mice with gko deficient live just 15±1d and have 10× to100× virus compared with the control. There are necrosis of spleen, liver and lung in the gko-knockout mice. After the injection of exogenous IFNγ can heighten the defense and prolong their survival without recovery absolutely. Macrophages activation by IFNγ is proved a resolution of tuberculosis. They have previously reported that NO, and its related gene RNI, are responsible for destruction of virulent tubercle bacilli by murine macrophages, a response that requires treatment in vitro with both IFNγ and TNFα. So that production of RNI, at least in the murine model, may be a necessary mechanism for the control of tuberculosis infection. What’s more, findings in the gko model may have relevance to tuberculosis in AIDS[8].
IFN gamma and Tolerance
IFNγ is one of the most important proinflammatory factor as it can induce the production of Th1 which initiates and maintains the inflammatory process in affected organs. To day, there are amounts of reports which associate IFNγ with immune tolerance induction, both in vitro and in vivo. It seems that these effects, either pro-inflammatory or tolerogenic, are largely dependent on the specific immunological setting and the timeline of the immune response. Dendritric cells(DCs) exerts atigen sampling and messages delivering to responding T cells originally. IFNγ can be produced by DCs and IFNγ can induce DCs activation and tolerance in some scenarios. Treatment of DCs with high doses IFNγ induces the expression of IDO which has an immunorepressive function leading to inhibiting effector T cells, and its long-term maintenance is shown to be supported by kynurenine-aryl hydrocarbon (AhR) pathway. IFNγ seems to be paradoxical that IFNγ sometimes aggravates the severity of autoimmune disease and attenuates it in others[9].
Interferon and Antiretroviraling Restriction Factors
Antiviral restriction factors is the vanguard in the defense line but it is constitutively expressed low in many cell types which should be enhanced encountering pathogens. IFN and some interleukin do this job and establish the antiviral state. The definition of restriction factors is fuzzy as it is diverse with solid function. Restriction factors like SERINC3 and SERINC5 has antiviral function without general inflammation and side effects. Restriction factors like PRRs and TLRs are receptors to restrict the pattern recognition of pathogen. High levels of IFN transiently suppresses viral replication during the chronic phase of HIV-1 infection and the induction of restriction factors contributes to this effect[4].
Interferon and Multiple Sclerosis
Multiple sclerosis(MS) is a chronic and dysregulatory disease of the central nervous system. IFNβ is the first approved and still the most widely used to treat MS. When produced in high amounts, as typically seen in acute viral infections, IFNβ up-regulates anti-inflammatory cytokines, and downregulates the pro-inflammatory ones. IFNα has similar effects of IFNβ. On the contrary, IFNγ has the opposite effects that it further induces Th1 immune response leading to the development and relapse of MS[10].
Interferon and Premature Birth
Pretern birth causes many severe health problems in children under 5 years old due to the infection and inflammation. The inflammation mediates by additional interleukin, tumor necrosis factor and IFNγ will probably cause fetal membrane damage, uterine contraction and biochemical and structural changes in the cervix. What's more, newborns have weak and damaged innate and adaptive immune system with less IgG[11].
While IFNβ administration during early pregnancy seems to be pharmacologically safe and accumulating real-life evidence suggests that IFNβ therapies are not related to severe pregnancy problems. In consideration of these issues, IFNβ therapy might be continued in women with high risk of disease activity while trying to become pregnant. Additionally, IFNβ is not absorbed through the gastrointestinal tract, and available data suggests that the IFNβ milk levels attainable at usual doses are very low, hence, IFNβ administration seems to be safe for the baby[12][13][14].
Interferon and hepatitis C
Chronic hepatitis, caused by infection with hepatitis C virus C (HCV), also known as chronic hepatitis C (CHC), is parenterally transmitted and primarily through unsafe blood transfusions, the use of injected drugs and therapeutic injections. In humans, the only natural host for HCV is hepatocytes where the virus infects and replicates[15].
The type-1 IFNs include interferon α, β, ε, к, and ω in human. All type I IFNs have antiviral, antiproliferative and immunomodulatory activities, but their relative potencies are not different. Most forms of type I IFN have activity against HCV, yet few have been evaluated clinically[16]. Currently, the commercially available forms of IFNα used for hepatitis C (α2a, α2b and consensus IFN) have somewhat different potent in vitro but appear to yield similar response rates in treated patients. The type-1 IFNs might have similar clinical activities because they share the same, at least in part, cell-surface receptors and intracellular pathways of action. As a crucial mediator of the innate antiviral immune response, interferon alpha(IFNα) was a natural choice for treatment[17].
5. The latest research of Interferon
In this part, we are listing some latest researches about interferon.
#1 The research of Elitza S. Theel et al. suggested that the necessary transition to the QFT-Plus assay would be associated with a minimal difference in assay performance characteristics though comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube Interferon-γ eelease assays in Patients at Risk for Tuberculosis and in healthcare workers. Please click here to obtain the article.
#2 Li F et al. demonstrated that mice deficient in E3 ligase gene Hectd3 remarkably increased host defense against infection by intracellular bacteria F. novicida, Mycobacterium, and Listeria by limiting bacterial dissemination. In the absence of HECTD3, type I IFN response was impaired during bacterial infection both in vivo and in vitro. These results indicated that HECTD3 mediated TRAF3 polyubiquitination and type I interferon induction during bacterial infection. Please click here to obtain the article.
#3 Pulmonary, a researcher from critical care & sleep medicine of department of medicine in state university of New York, found that macrophages could be effectively immune-stimulated by aerosol therapy by repurposing IFN γ as an inhaled aerosol and targeting directly to the lung to treat a host of diseases affected by dysregulated immunity.Please click here to obtain the article.
References
[1] Ng, C.T., Sullivan, B.M., et al. Blockade of Interferon Beta, but Not Interferon Alpha, Signaling Controls Persistent Viral Infection[J]. Cell Host Microbe.2015, 17, 653-661.
[2] Cherie T. Ng,1 Juan L. Mendoza, et al. Alpha and Beta Type 1 Interferon Signaling: Passage for Diverse Biologic Outcomes[J]. Cell. 2016, 164, 349-352.
[3] Nan Y, Wu C, et al. Interferon independent non-canonical STAT activation and virus induced inflammation[J]. Viruses, 2018(4): Apr.
[4] Kathrin S, Julia D, et al. Interferon α subtypes in HIV infection[J]. Cytokines Growth Factor Rev, 2018 Feb.
[5] Leonidas C. Platanias. Mechanisms of type I and type II interferon mediated signaling[J]. Nat Rev Immunol.2005 5(5):375-86.
[6] Aqbi HF, Wallace M, et al. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression[J]. J Leukoc Biol, 2018 Feb.
[7] Hotter D, Kirchhoff F. Interferons and beyond: Induction of antiretroviral restriction factors[J]. J Leukoc Biol, 2018(3): 465-477.
[8] Flynn JL, Chan J, et al. An essential role for interferon γ in resistance to mycobacterium tuberculosis infection[J]. J Exp Med, 1993(6): 2249-2254.
[9] Rozman P, Svajger U. The tolerogenic role of IFN-γ[J]. Cytokines Growth Factor Rev, 2018 Apr
[10] Dumitrescu L, Constantinescu CS, et al. Recent developments in interferon-based therapies for multiple sclerosis[J]. Expert Opin Biol Ther, 2018 Apr.
[11] Helmo FR, Alves EAR, et al. Intrauterine infection, immune system and premature birth[J]. J Matern Fetal Neonatal Med, 2018(9): 1227-1233.
[12] Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs[J]. CNS Drugs, 2015(3): 207-220.
[13] Almas S, Vance J, et al. Management of multiple sclerosis in the breastfeeding Mother[J]. Mul Scler Int, 2016: 6527458.
[14] Hale TW, Siddiqui AA, et al.Transfer of interferon beta-1a into human breastmilk[J]. Breastfeed Med, 2012 (2):123-125.
[15] Shepard, C. W., et al. Global epidemiology of hepatitis C virus infection[J]. Lancet Infect. 2005, 5, 558-567.
[16] Robek, M. D., Boyd, et al. Lambda interferon inhibits hepatitis B and C virus replication[J]. J. Virol. 2005, 79, 3851-3854.
[17] Jordan J. Feld and Jay H. Hoofnagle. Mechanism of action of interferon and ribavirin in treatment of hepatitis C[J]. Nature.2005, 436(7053):967-72.







Comments
Leave a Comment