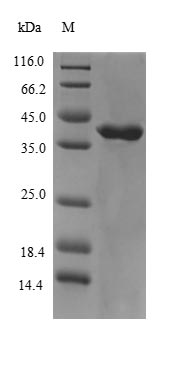
This recombinant human muscarinic acetylcholine receptor M3 (CHRM3) features a purity greater than 90%, as measured by SDS-PAGE. It is expressed from the expression vector carrying the gene encoding the 253-492aa of human CHRM3 and the N-terminal 6xHis-B2M-tag gene in E. coli. It is available in both liquid and lyophilized forms.
The human CHRM3 is involved in several critical biological processes, including glandular secretion, smooth muscle contraction, and modulation of insulin secretion from pancreatic islets [1][2]. It is predominantly expressed in tissues such as the brain, lungs, and gastrointestinal tract, where it contributes to regulating autonomic functions and influences processes like inflammation and cell proliferation. In the pancreas, CHRM3 is crucial in enhancing glucose-induced insulin secretion. Studies have demonstrated that stimulation of the CHRM3 leads to increased insulin release, which is essential for maintaining glucose homeostasis [1][3]. CHRM3's activation is mediated through phosphorylation processes that enhance its signaling capabilities, particularly in response to agonists like acetylcholine and pilocarpine [4][5].
Dysregulation of CHRM3 has been implicated in various pathophysiological conditions, including asthma, obesity, and certain neurodegenerative diseases. The activation of CHRM3 can promote tumor cell proliferation and migration, particularly in small-cell lung carcinoma and gastric cancer [6][7]. The CHRM3 signaling involves various intracellular pathways, including the activation of protein kinase D1 and the mitogen-activated protein kinase (MAPK) pathway, which are crucial for mediating the effects of M3 receptor activation on cell proliferation and insulin secretion [1][3][8].
References:
[1] K. Kong, A. Butcher, P. McWilliams, D. Jones, J. Wess, F. Hamdanet al., M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase d1, Proceedings of the National Academy of Sciences, vol. 107, no. 49, p. 21181-21186, 2010. https://doi.org/10.1073/pnas.1011651107
[2] T. Renuka, R. Robinson, & C. Paulose, Increased insulin secretion by muscarinic m1 and m3 receptor function from rat pancreatic islets in vitro, Neurochemical Research, vol. 31, no. 3, p. 313-320, 2006. https://doi.org/10.1007/s11064-005-9022-6
[3] A. Duttaroy, C. Zimliki, D. Gautam, Y. Cui, D. Mears, & J. Wess, Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor–deficient mice, Diabetes, vol. 53, no. 7, p. 1714-1720, 2004. https://doi.org/10.2337/diabetes.53.7.1714
[4] A. Butcher, R. Prihandoko, K. Kong, P. McWilliams, J. Edwards, A. Bottrillet al., Differential g-protein-coupled receptor phosphorylation provides evidence for a signaling bar code, Journal of Biological Chemistry, vol. 286, no. 13, p. 11506-11518, 2011. https://doi.org/10.1074/jbc.m110.154526
[5] I. Torrecilla, E. Spragg, B. Poulin, P. McWilliams, S. Mistry, A. Blaukatet al., Phosphorylation and regulation of a g protein–coupled receptor by protein kinase ck2, The Journal of Cell Biology, vol. 177, no. 1, p. 127-137, 2007. https://doi.org/10.1083/jcb.200610018
[6] P. Song, H. Sekhon, A. Lu, J. Arredondo, D. Sauer, C. Gravettet al., M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion, Cancer Research, vol. 67, no. 8, p. 3936-3944, 2007. https://doi.org/10.1158/0008-5472.can-06-2484
[7] L. Wang, J. Xu, Y. Xia, K. Yin, Z. Li, B. Liet al., Muscarinic acetylcholine receptor 3 mediates vagus nerve-induced gastric cancer, Oncogenesis, vol. 7, no. 11, 2018. https://doi.org/10.1038/s41389-018-0099-6
[8] T. Takahashi, A. Shiraishi, J. Murata, S. Matsubara, S. Nakaoka, S. Kirimotoet al., Muscarinic receptor m3 contributes to intestinal stem cell maintenance via ephb/ephrin-b signaling, Life Science Alliance, vol. 4, no. 9, p. e202000962, 2021. https://doi.org/10.26508/lsa.202000962