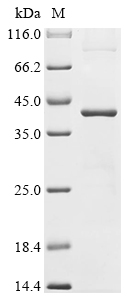
To produce the recombinant human NPM1 protein with an N-terminal 6xHis tag, the NPM1 gene (1-294aa) is co-cloned into a vector with the 6xHis-tag gene. The E. coli cells are transformed with the recombinant vector, and protein expression is induced using IPTG. After cell lysis, the recombinant NPM1 protein is purified using affinity chromatography. Its purity is verified using SDS-PAGE, and the final product shows over 90% purity, confirming its suitability for biochemical assays.
Human NPM1 is a multifunctional nucleolar phosphoprotein that plays critical roles in various cellular processes, including ribosome biogenesis, cell cycle regulation, and apoptosis. It is primarily localized in the nucleolus but can shuttle between the nucleus and cytoplasm, reflecting its diverse functional roles in cellular homeostasis and stress response [1][2].
NPM1 is involved in the assembly and transport of ribosomal components, which is essential for protein synthesis. Its expression is often upregulated in proliferating cells, indicating its potential role in promoting cell growth and division [1][2]. Moreover, NPM1 has been implicated in the regulation of oncogenic processes. It interacts with several key proteins, including c-Myc and p53, and has been shown to influence their activities, thereby affecting cell proliferation and tumorigenesis [3][4]. Overexpression of NPM1 has been associated with poor prognosis in various cancers, including gastric, breast, and colorectal cancers, where it contributes to tumor growth and resistance to apoptosis [5][6][7].
NPM1 is also involved in cellular responses to DNA damage and stress. It has been shown to participate in the repair of DNA and the regulation of cell survival pathways, which further underscores its importance in maintaining cellular integrity [8][9]. The protein's ability to shuttle between the nucleolus and cytoplasm allows it to respond dynamically to cellular signals, making it a critical player in the cellular stress response [10].
References:
[1] B. Yung, C‐myc‐mediated expression of nucleophosmin/b23 decreases during retinoic acid‐induced differentiation of human leukemia hl‐60 cells, Febs Letters, vol. 578, no. 3, p. 211-216, 2004. https://doi.org/10.1016/j.febslet.2004.08.089
[2] D. Stępiński, The nucleolus, an ally, and an enemy of cancer cells, Histochemistry and Cell Biology, vol. 150, no. 6, p. 607-629, 2018. https://doi.org/10.1007/s00418-018-1706-5
[3] Z. Li, D. Boone, & S. Hann, Nucleophosmin interacts directly with c-myc and controls c-myc-induced hyperproliferation and transformation, Proceedings of the National Academy of Sciences, vol. 105, no. 48, p. 18794-18799, 2008. https://doi.org/10.1073/pnas.0806879105
[4] J. Wong, M. Hasan, M. Rahman, A. Yu, S. Chan, D. Schaeffer, et al. Nucleophosmin 1, upregulated in adenomas and cancers of the colon, inhibits p53‐mediated cellular senescence, International Journal of Cancer, vol. 133, no. 7, p. 1567-1577, 2013. https://doi.org/10.1002/ijc.28180
[5] F. Zhou, E. Chen, D. You, Y. Song, Z. Sun, & L. Yue, Both high expression of nucleophosmin/b23 and crm1 predicts poorer prognosis in human gastric cancer, Apmis, vol. 124, no. 12, p. 1046-1053, 2016. https://doi.org/10.1111/apm.12604
[6] J. Yun, M. Jiang, G. Chen, Q. Tian, C. Zhang, J. Xiang, et al. Increased expression of nucleophosmin/b23 in hepatocellular carcinoma and correlation with clinicopathological parameters, British Journal of Cancer, vol. 96, no. 3, p. 477-484, 2007. https://doi.org/10.1038/sj.bjc.6603574
[7] C. Luo, T. Lei, M. Zhao, Q. Meng, & M. Zhang, Cd40 is positively correlated with the expression of nucleophosmin in cisplatin-resistant bladder cancer, Journal of Oncology, vol. 2020, p. 1-10, 2020. https://doi.org/10.1155/2020/3676751
[8] I. Kalousek, P. Otevřelová, & P. Röselová, Expression and translocation of major nucleolar proteins in relation to the transcriptional activity of the nucleolus, Journal of Applied Biomedicine, vol. 3, no. 4, p. 175-186, 2005. https://doi.org/10.32725/jab.2005.023
[9] M. Wu, J. Chang, C. Chou, & B. Yung, Involvement of nucleophosmin/b23 in the response of hela cells to uv irradiation, International Journal of Cancer, vol. 97, no. 3, p. 297-305, 2001. https://doi.org/10.1002/ijc.1606
[10] S. Duangmano, P. Sae-lim, A. Suksamrarn, F. Domann, & P. Patmasiriwat, Cucurbitacin b inhibits human breast cancer cell proliferation through disruption of microtubule polymerization and nucleophosmin/b23 translocation, BMC Complementary and Alternative Medicine, vol. 12, no. 1, 2012. https://doi.org/10.1186/1472-6882-12-185