Thanks for your inquiry.
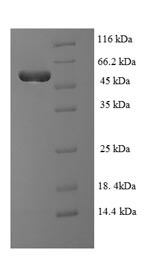
Recombinant Human TAR DNA-binding protein 43(TARDBP),partial
CSB-EP023129HU >> E.coli N-terminal 6xHis-SUMO-tagged
CSB-EP023129HUe0 >> E.coli N-terminal GST-tagged
Expression Region:1-396aa; Fragment at the N-terminal.
Sequence:
MSEYIRVTEDENDEPIEIPSEDDGTVLLSTVTAQFPGACGLRYRNPVSQCMRGVRLVEGILHAPDAGWGNLVYVVNYPKDNKRKMDETDASSAVKVKRAVQKTSDLIVLGLPWKTTEQDLKEYFSTFGEVLMVQVKKDLKTGHSKGFGFVRFTEYETQVKVMSQRHMIDGRWCDCKLPNSKQSQDEPLRSRKVFVGRCTEDMTEDELREFFSQYGDVMDVFIPKPFRAFAFVTFADDQIAQSLCGEDLIIKGISVHISNAEPKHNSNRQLERSGRFGGNPGGFGNQGGFGNSRGGGAGLGNNQGSNMGGGMNFGAFSINPAMMAAAQAALQSSWGMMGMLASQQNQSGPSGNNQNQGNMQREPNQAFGSGNNSYSGSNSGAAIGWGSASNAGSGSG
Sorry, we haven't detected the activity of this protein, so we can't 100% guarantee its activity, however, the proteins expressed by E.coli and Yeast expression systems have been ordered many times by customers through the worldwide, and it's supernatant expression, so it has great potential to be active.