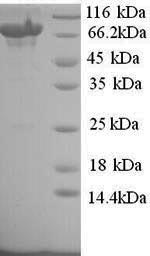
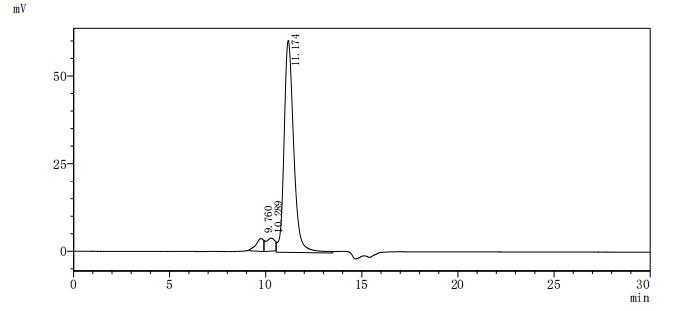
CUSABIO's product CSB-YP023607HU was recombinantly expressed in yeast, with an N-terminal s6xHis-tag. Its expression region corresponds to the 27-827aa of the human TLR8 protein. The purity of this recombinant human TLR8 protein is greater than 90% as measured by SDS-PAGE.
Human TLR8 is expressed in human monocytes, macrophages, neutrophils, myeloid dendritic cells, and regulatory T cells [1]. TLR8 induces cytokine production in response to specific ligands, contributing to the production of inflammatory cytokines in conditions like rheumatoid arthritis and systemic sclerosis [2]. Additionally, TLR8 has been shown to inhibit the activation of TLR7 and TLR9, highlighting its regulatory functions within the immune response [3].
Studies have demonstrated that TLR8 is a sensor for bacterial RNA in human monocytes, particularly in response to Gram-positive bacteria like Streptococcus pyogenes, Staphylococcus aureus, and Streptococcus pneumonia [4][5]. Furthermore, TLR8 activation has been linked to the early detection of bacteria, induction of inflammation, and recruitment of leukocytes to sites of infection [6]. The importance of TLR8 in recognizing pathogens is further emphasized by its ability to enhance the protective efficacy of immunization against infections like Mycobacterium tuberculosis [7]. TLR8 has also been associated with epigenomic remodeling and inflammatory response regulation through signaling pathways involving TBK1-IRF5 [2].
References:
[1] N. Ishii, K. Funami, M. Tatematsu, T. Seya, & M. Matsumoto, Endosomal localization of tlr8 confers distinctive proteolytic processing on human myeloid cells, The Journal of Immunology, vol. 193, no. 10, p. 5118-5128, 2014. https://doi.org/10.4049/jimmunol.1401375
[2] Y. Liu, M. Bachu, C. Brauner, R. Yuan, Y. Du, M. Kioonet al., Costimulation of tlr8 responses by cxcl4 in human monocytes mediated by tbk1-irf5 signaling and epigenomic remodeling, The Journal of Immunology, vol. 208, no. 1_Supplement, p. 111.01-111.01, 2022. https://doi.org/10.4049/jimmunol.208.supp.111.01
[3] J. Cervantes, B. Weinerman, C. Basole, & J. Salazar, Tlr8: the forgotten relative revindicated, Cellular and Molecular Immunology, vol. 9, no. 6, p. 434-438, 2012. https://doi.org/10.1038/cmi.2012.38
[4] T. Eigenbrod, K. Pelka, E. Latz, B. Kreikemeyer, & A. Dalpke, Tlr8 senses bacterial rna in human monocytes and plays a nonredundant role for recognition of streptococcus pyogenes, The Journal of Immunology, vol. 195, no. 3, p. 1092-1099, 2015. https://doi.org/10.4049/jimmunol.1403173
[5] S. Moen, B. Ehrnström, J. Kojen, M. Yurchenko, K. Beckwith, J. Afsetet al., Human toll-like receptor 8 (tlr8) is an important sensor of pyogenic bacteria, and is attenuated by cell surface tlr signaling, Frontiers in Immunology, vol. 10, 2019. https://doi.org/10.3389/fimmu.2019.01209
[6] B. Ehrnström, J. Kojen, M. Giambelluca, L. Ryan, S. Moen, Z. Huet al., Tlr8 and complement c5 induce cytokine release and thrombin activation in human whole blood challenged with gram-positive bacteria, Journal of Leukocyte Biology, vol. 107, no. 4, p. 673-683, 2020. https://doi.org/10.1002/jlb.3a0120-114r
[7] J. Tang, M. Sun, G. Shi, Y. Xu, Y. Han, X. Liet al., Toll-like receptor 8 agonist strengthens the protective efficacy of esat-6 immunization to mycobacterium tuberculosis infection, Frontiers in Immunology, vol. 8, 2018. https://doi.org/10.3389/fimmu.2017.01972