What Is the Toll-like Receptor?
Toll-like receptors (TLRs), named after the TOLL protein in Drosophila, are one type of cellular pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs). TLRs are membrane-bound receptors and are responsible for detecting microbial pathogens and generating innate immune responses.
There are 12 TLR receptors in mice, TLR1~9 and TLR11~13, while TLR1~10 in humans. All of these TLR receptors are membrane-bound receptors that contain a cytoplasmic Toll-IL-1 receptor (TIR) domain, which can interact with signaling ligands.
Mammalian TLRs are expressed on innate immune cells, such as macrophages and dendritic cells, and respond to the membrane components of Gram-positive or Gram- negative bacteria. Pathogen recognition by TLRs provokes rapid activation of innate immunity by inducing the production of pro-inflammatory cytokines and up-regulation of co-stimulatory molecules.
What Is the Toll-like Receptor Signaling Pathway?
Each TLR binds to specific ligands, initiates a tailored innate immune response to the specific class of pathogen, activating the adaptive immune response. The process is called the Toll-like Receptor signaling pathway.
The Function of Toll-like Receptor Signaling Pathway
Mammalian TLRs play an important role in recognizing and removing pathogenic microorganisms, mediating the production of downstream cytokines, and linking innate and acquired immunity.
The Mechanism of Toll-like Receptor Signaling Pathway
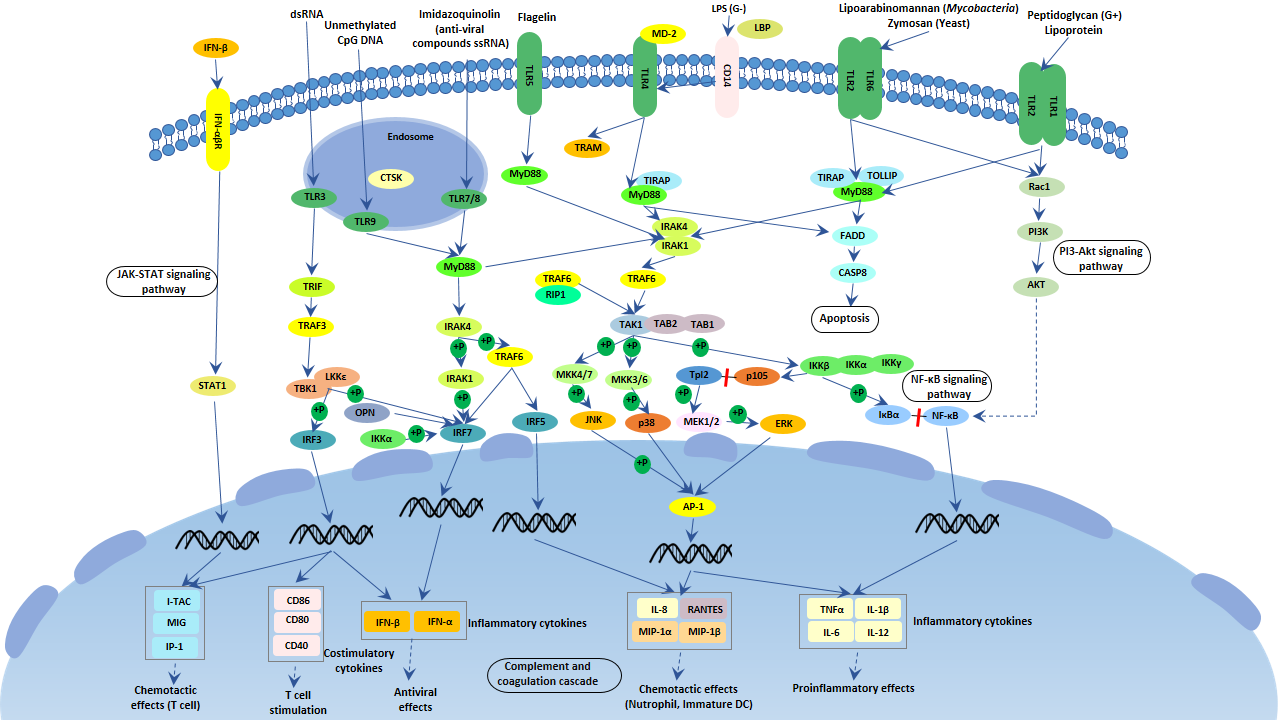
With deep research, TLR signaling pathways are separated into two groups: a MyD88-dependent pathway that leads to the production of pro-inflammatory cytokines with the quick activation of NF-kappa B and MAPK, and a MyD88-independent pathway associated with the induction of IFN-β & IFN-inducible genes and maturation of dendritic cells with slow activation of NF-kappa B & MAPK.
TLRs are mostly located on the cell membrane and mainly sense extracellular pathogen components. TLRs mediated signaling pathways exert functions through their TIR domains. The TIR domains of these receptors (except the TLR3 receptor) can interact with the adapter MyD88 protein. The MyD88 protein further recruits IRAK family proteins. IRAK4 protein recruited into the receptor complex can phosphorylate and activate IRAK1 protein and IRAK2 protein, which promotes the oligomerization of TRAF6 protein, activating the ubiquitin ligase activity of TRAF6. TRAF6 protein, together with the E2 binding enzyme complex UBC13-UEV1A, can catalyze the polyubiquitination, activating TAK1, IKK, and MAPK.
TLR3 and TLR4 interact with TRIF by binding to their respective ligands, double-stranded RNA and lipopolysaccharide, respectively. TRIF subsequently links to TRAF6 and RIP1 proteins, activating the NF-κB signaling pathway. TRIF proteins also bind to IKK-like kinases TBK1 and IKK-ɛ, phosphorylating their substrate IRF3 protein. The phosphorylated IRF3 protein forms a dimer, which enters the nucleus and assembles into an enhancer complex, together with the NF-κB protein and activated transcription factor 2 (ATF2) form. The enhancer complex can induce the expression of IFN-β. The TLR4 signaling pathway has been well studied.
Some other TLRs such as TLR7, TLR8, and TLR9 can also induce IFN-I expression. TLR7 and TLR8 bind to viral single-stranded RNA, while TLR9 binds to unmethylated CpG DNA. After binding to the ligands, they can recruit cytoplasmic signal complexes composed of MyD88, IRAKs, and TRAF6 in the cytoplasm.





