The immune system can accurately identify "self" and "non-self", make the body produce protective immune response and realize the monitoring of tumor.
Treg cells are regulatory T cells, which are the basic cells in the immune system, which was discovered by Sakaguchi et al. [1] in 1995.
Treg cells are characterized by expressing Foxp3, CD25 and CD4. It belongs to a class of CD4+ T cell subsets with low proliferation ability. It plays an important role in immune homeostasis and induction of immune tolerance.
1. Classification of Treg Cells
According to its origin, regulatory T cells can be further divided into natural regulatory T cells (nTreg) and adaptive or inducible regulatory T cells (aTreg or iTreg).
nTreg is mainly CD4+Treg cells, which are differentiated in the thymus by progenitor cells from bone marrow, accounting for about 1% - 3% of the total number of CD4+T lymphocytes, accounting for 5% of the total number of CD4+T lymphocytes in peripheral blood [2]. It plays an immunomodulatory role in peripheral blood, lymphatic organs, inflammatory sites and tumor tissues [3].
aTreg or iTreg include: Th3 (phenotypic characteristic is CD4+CD25low), Tr1 (phenotypic characteristic is CD4+CD25lowCD45RBlow), CD8+ regulatory T cell, natural killer T cell (NKT) and other subtypes. They are closely related to the occurrence of autoimmune diseases and tumors.
iTreg also known as sTreg, is a class of regulatory T cells derived from peripheral mature T cells stimulated by specific antigens and induced by immunosuppressive cytokines (mainly including TGF-β, IL-2, IL-10, IFN-γ, IFN-α, indoleamine 2-3 dioxygenase, and retinoic acid), which accounts for about 4% - 7% of the total number of CD4+T cells.
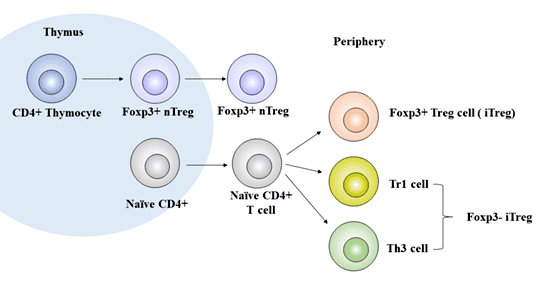
Figure 1 The development of Treg cells
2. Treg Cell Differentiation
The differentiation, development and function of Treg cells are regulated by a variety of cytokines. Transcription factor forkbox P3 (Foxp3) is involved in the differentiation. Transcriptional activator STAT5 is another important factor involved in the differentiation and survival of Treg cells.
The development of nTreg in the thymus is dependent on the synergistic stimulation of TCR and CD28, which is essential for the steady proliferation and survival of peripheral nTregs.
The development of iTreg requires IL-2 and transforming growth factor (TGF- β), rather than co-stimulation with CD28 [4].
Studies have shown that [5] IL-2 promotes the production of inducible regulatory T cells through STAT5, and IL-2 together with TGF-β induces the naive CD4+ CD25-T cells to transform into CD4+ CD25+ T cells and express Foxp3.
3. Treg Cell Marker
At present, it is believed that CD4+CD25+ Foxp3+ is the main phenotype of Treg cells.
Treg also lowly expressed another specific marker, CD127.
Some receptors are also expressed on the surface of Treg, such as CD5, CD38, CD45, CD62L, CD103, CTLA-4 and inhibitory immune receptor GITR.
The markers expressed by Treg cells can be divided into two categories according to their location:
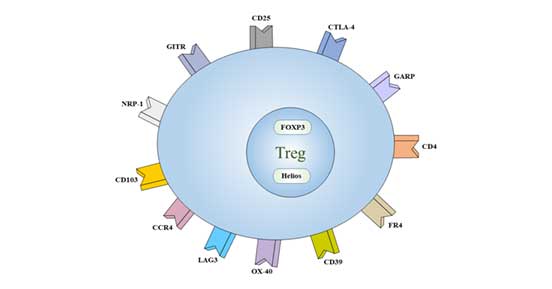
Figure 2 Cell markers of Treg
3.1 Intracellular Markers
FOXP3: Fontenot JD et al. [6] found that Treg cells highly express forkhead box P3 (Foxp3), which can promote the transformation of immature CD4+T cells into Treg [7].
FOXP3 is a member of the forkhead-like transcription factor family, which is related to the regulation of cell growth and development. FOXP3 is closely related to Treg cells. If FOXP3 gene mutation occurs, it will affect the development and maturation of Treg cells and cause some diseases.
FOXP3 is mainly expressed in lymphoid organs and tissues such as thymus, spleen and lymph nodes. At present, Foxp3 is recognized as the most sensitive marker of Treg cells.
Helios: The gene encodes a member of the Ikaros family of zinc finger proteins and is a hematopoietic specific transcription factor involved in the regulation of lymphocyte development. This protein forms homotype or heteromeric dimers with other members of the Ikaros family and is thought to play a major role in early hematopoietic development.
3.2 Treg Cell Surface Markers
CD4: CD4, also known as T4/leu-3, is a member of the immunoglobulin superfamily. It is a single chain I transmembrane glycoprotein with a molecular weight of 55 kDa.
CD4 β is a part of TCR/CD3 complex and participates in TCR signal transduction.
It is expressed in most thymocytes, helper T cells, type II NKT cells and monocytes / macrophages.
CD25: CD25 is also called IL-2R α. Ly-43, P55 or Tac, is a kind of glycoprotein with molecular weight of 55kDa. It is expressed on activated T and B cells, thymocyte subsets, pre-B cells and Treg cells.
CD39: CD39 (nucleoside triphosphate diphosphohydrolase-1, NTPDase 1) is an extracellular enzyme that can degrade ATP to AMP. It is expressed in B cells, dendritic cells and T cell subsets including regulatory T cells and memory T cells. CD39 is a major member of the immune system, involved in the inhibition of inflammation and the control of platelet activation.
CD62L: CD62L, called L-selectin or LECAM-1, is a single chain type I glycoprotein with a molecular weight of 74 - 95 kDa. It is expressed on most peripheral blood B cells, T cells, NK cell subsets, monocytes, granulocytes and some malignant cells of hematopoietic system. CD62L is very important for immature lymphocytes to homing to high endothelial venules in peripheral lymph nodes and Peyer's plaques.
CD73: CD73 is a cell surface protein anchored to cells by GPI, with a molecular weight of 69 kDa. In mice, the expression of CD73 in the bone marrow was limited to CD11b+ myeloid cells. In the spleen, it is expressed primarily on T cells.
CD103: CD103, also known as α E integrin or integrin α IEL chain, belongs to the integrin family and is a type I transmembrane glycoprotein. Treg cells highly express CD103. CD103 binds to E-cadherin and mediates lymphocyte homing to intestinal epithelial cells.
CD134: CD134 is a member of the TNF receptor family, also known as OX40 and TNFRSF4, is a 50 kDa type I transmembrane glycoprotein. OX40 was expressed on activated T lymphocytes. The interaction between OX40 and OX40L leads to B cell proliferation and antibody secretion, and regulates primary T cell proliferation and T cell survival. OX40 affects the regulation of tolerance of CD4+T cells.
CD152 (CTLA-4): CD152 is a member of the immunoglobulin superfamily, also known as CTLA-4 or Ly-56, with a molecular weight of 33 kDa. It is expressed on activated T and B lymphocytes.
CTLA-4 negatively regulates cell-mediated immune response, which plays a role in inducing and maintaining immune tolerance, developing protective immunity and regulating thymocytes.
CD194 (CCR4): CCR4 ligands include: CCL17 (TARG) and CCL22 (MDC). CCR4 is expressed in memory T cells, macrophages, platelets, basophils, Th2 cells and Treg cells.
CCR4 and its ligands (CCL17 and CCL22) play an important role in the recruitment of memory T cells in various skin immune diseases.
FR4: Folate receptor 4 (FR4) is the surface receptor of folic acid (vitamin B9). It has high constitutive expression on mouse CD4+ CD25+ natural regulatory T cell (Treg). It binds to CD4 and CD25 and distinguishes Treg from other types of T cells.
GARP: GARP, also known as leucine-rich repeat sequence 32 (LRC32), is a type I membrane glycoprotein with molecular weight of 80kDa. GARP exists on the surface of megakaryocytes, platelets and activated Treg (CD4+, CD25+, FoxP3+ cells), and is a receptor for transforming growth factor-β (TGF-β). GARP may play a role in controlling the inhibitory function of Tregs.
GITR: GITR (glucocorticoid-induced TNFR-related genes), also known as TNFRSF18 and AITR, is a members of the TNF receptor superfamily. It is highly expressed on CD25+CD4+Tregs. The interaction between GITR and its ligands can enhance T cell activation, proliferation and cytokine production, and eliminate the inhibitory function of CD25+CD4+Tregs. The activation of GITR in vivo leads to the development of autoimmune diseases and the restoration of suppressed immune responses.
TGF-β: TGF-β is a potent stimulator of osteoblast formation and plays an important role in bone remodeling.
It can regulate the lineage differentiation of Th17 cells or Treg cells. High concentration is beneficial to the development of Treg cells. The synergistic action of low concentration of TGF- β with IL-6 and IL-21 is beneficial to the differentiation of Th17 cells. It also controls cell proliferation, differentiation and other functions in many cell types.
CD127: CD127, also known as IL-7Rα, is a type I transmembrane glycoprotein with a molecular weight of 60 kDa. The expression of CD127 is down-regulated in Treg cells, and the lack of CD127 is one of the characteristics of Tregs cells. It can be used as a marker of Treg and routine T cell differentiation.
4. What do Treg Cells Do?
The general physiological functions of Treg mainly include the following aspects:
Regulatory T Cells and Immune Tolerance: By inhibiting self-reactive T cells, Treg enables the body to develop active tolerance to its own antigens, preventing the occurrence of autoimmune diseases. In tumors, Treg makes the body produce antigenic tolerance to tumors through immunosuppression, which makes tumor cells escape the immune killing of the body.
Promote Chronic Inflammatory Response: When pathogens invade, effector T cells clear pathogens through a series of immune responses, while Treg plays an opposite role with other immune cells in the body [8]. It exerts inhibitory functions by the secretion of cytokines such as IL-4, IL-10 and TGF-β. It can prevent the occurrence of pathological immune response that causes tissue destruction, but at the same time, it also makes it difficult to remove pathogens and prolong the course of chronic infection.
Immunosuppression: the main function of Treg is to negatively regulate the immune response of the body, so Treg plays a vital role in regulating immune homeostasis and preventing the occurrence of autoimmune diseases. Through immunosuppression, Treg promotes tumor immune escape [9], so it is also regarded as a kind of immune cell that helps tumor survive and promote its growth.
Treg cells regulate immune function and participate in the aging process of the human body. The immune function of mice decreased during aging.
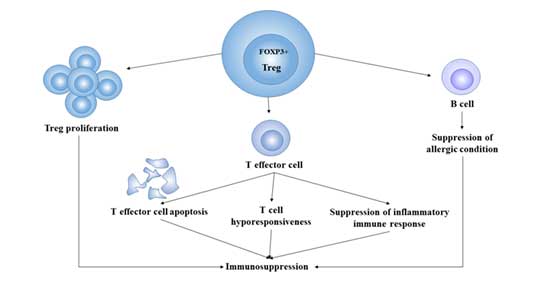
Figure 3 Immunosuppression of Treg cells
5. How do Treg Cells Work?
Treg exerts its immunomodulatory function in two ways:
5.1 Direct Cell-to-Cell Contact
Some chemokines cause Treg to gather around immune cells and play a role through a cell-to-cell contact-dependent mechanism. Treg can directly bind to the corresponding receptors on the target cells through CTLA-4, TGF-β and GITR, and inhibit the proliferation of immune cells such as CD4+T, CD8+T, dendritic cells and antigen presenting cells [10].
Treg inhibits the immune response by regulating the number and activity of dendritic cells to invalidate their antigen presentation.
5.2 Secretion of Inhibitory Cytokines
Treg negatively regulates immunity by secreting inhibitory cytokines, such as IL-4, IL-10, IL-35 and TGF- β [11] [12].
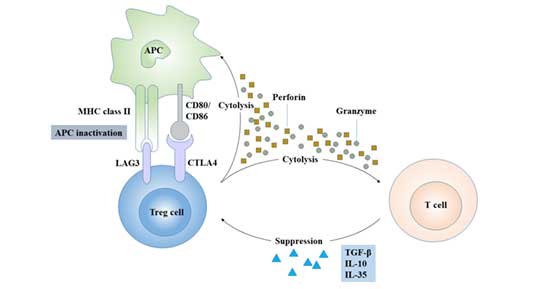
Figure 4 Regulatory mechanism of Treg cells
6. Regulatory T Cells in Cancer
Tumor microenvironment plays a very important role in the occurrence and development of tumor. Immune cells can affect tumor progression by affecting tumor microenvironment.
Generally speaking, most immune cells can play the role of anti-tumor immunity. Such as helper T cells and cytotoxic T cells. However, Treg plays an opposite role in tumor microenvironment. The immunosuppressive effect of Treg can not only prevent the occurrence of autoimmune diseases, but also promote the immune escape of tumor cells, indirectly accelerate the proliferation of tumor cells and enhance the infiltration ability of tumor cells.
Treg cells can inhibit the development and activation of effector cells and play an important role in mediating tumor immune tolerance. Studies have shown that the number of Treg cells is negatively correlated with the prognosis of tumors [13].
Tumor immune tolerance induced by Treg cells is realized by controlling primary T cells and memory T cells.
Treg cells can also influence CD4+ (Th1, Th2, Th17, NK) and other immune cells through TGF-β induce host immune tolerance.
The immune tolerance induced by Treg cells is also related to the role of dendritic cells (DC) [14].
7. Treg Cells and Immunotherapy
Treg cells play an immunosuppressive role in the immunity of the body, which is favored in the treatment of bone marrow transplantation.
In patients with multiple myeloma, low lymphatic status can improve the success rate of bone marrow transplantation.
Treg cells mediate the tolerance of antigens in the body. The decrease of its number will decrease the tolerance of the body to some of its own antigens and increase the rejection of grafts. Therefore, stimulating the recovery and regeneration of Treg cells in the human body can significantly reduce the rejection reaction of patients to the graft and greatly improve the success of bone marrow transplantation.
The immunosuppressive effect of Treg cells is related to immune escape and tolerance of tumor antigens. Therefore, in tumors, how to reduce the function of Treg is the key.
IL-2 is essential for the development of Treg cells [15]. IL-21 is a kind of cytokine which is similar to IL-2, but has no immunomodulatory function. Replacing IL-2 with IL-21 can prevent Treg cells from developing into sTreg cells. In addition, the combination of anti-CD25+ monoclonal antibody and anti-CD4+ monoclonal antibody can block Treg cells in vivo and eliminate the effect of Treg cells to the maximum extent. Experiments in mice have shown that the more thoroughly the Treg cells are removed, the better the effect of tumor immunotherapy is, and the longer the survival time of mice is [16].
Another idea of tumor immunotherapy is to reverse the immune tolerance induced by Treg cells to tumor cells.
Kiniwa et al. [17] found that TLR8 ligand (human Toll-like receptor 8 ligand) can reverse the immune anergy of Treg cells to tumor, eliminate the immune escape of tumor, and improve the efficiency of effector cells.
Other costimulatory factors B7.1 and B7.2 can also reverse the immune tolerance of Treg cells to tumor, eliminate the inhibition of effector cells, and improve the killing effect. Studies have found that when there is a lack of costimulatory factors B7.1 and B7.2, tumor cells will escape the immune surveillance of the body, making T cells in a state of incompetence or inducing their apoptosis, resulting in unlimited tumor growth.
References
[1] Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases [J]. The Journal of Immunology, 1995, 155(3): 1151-1164.
[2] Gavin M A, Rasmussen J P, Fontenot J D, et al. Foxp3-dependent programme of regulatory T-cell differentiation [J]. Nature, 2007, 445(7129): 771.
[3] Feuerer M, Hill J A, Mathis D, et al. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes [J]. Nature immunology, 2009, 10(7): 689.
[4] Cassis L, Aiello S, Noris M. Natural versus adaptive regulatory T cells [M]. Kidney Transplantation: Strategies to Prevent Organ Rejection. Karger Publishers, 2005, 146: 121-131.
[5] Zheng S G, Wang J, Wang P, et al. IL-2 is essential for TGF-β to convert naive CD4+ CD25− cells to CD25+ Foxp3+ regulatory T cells and for expansion of these cells [J]. The Journal of Immunology, 2007, 178(4): 2018-2027.
[6] Trzonkowski P, Szmit E, Myśliwska J, et al. CD4+ CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction [J]. Clinical immunology, 2004, 112(3): 258-267.
[7] Li Z, Li D, Tsun A, et al. FOXP3+ regulatory T cells and their functional regulation [J]. Cellular & molecular immunology, 2015, 12(5): 558.
[8] Klabusay M. The role of regulatory T-cells in antitumor immune response [J]. Klinicka onkologie: casopis Ceske a Slovenske onkologicke spolecnosti, 2015, 28: 4S23-7.
[9] Halvorsen E C, Mahmoud S M, Bennewith K L. Emerging roles of regulatory T cells in tumour progression and metastasis [J]. Cancer and Metastasis Reviews, 2014, 33(4): 1025-1041.
[10] Schlößer H A, Theurich S, Shimabukuro-Vornhagen A, et al. Overcoming tumor-mediated immunosuppression [J]. Immunotherapy, 2014, 6(9): 973-988.
[11] Collison L W, Workman C J, Kuo T T, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function [J]. Nature, 2007, 450(7169): 566.
[12] Sakaguchi S, Wing K, Onishi Y, et al. Regulatory T cells: how do they suppress immune responses? [J]. International immunology, 2009, 21(10): 1105-1111.
[13] Beyer M, Schultze J L. Regulatory T cells in cancer [J]. Blood, 2006, 108(3): 804-811.
[14] Banerjee D K, Dhodapkar M V, Matayeva E, et al. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients [J]. Blood, 2006, 108(8): 2655-2661.
[15] Frumento G, Piazza T, Di Carlo E, et al. Targeting tumor-related immunosuppression for cancer immunotherapy [J]. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders), 2006, 6(3): 223-237.
[16] El Andaloussi A, Han Y U, Lesniak M S. Prolongation of survival following depletion of CD4+ CD25+ regulatory T cells in mice with experimental brain tumors [J]. Journal of neurosurgery, 2006, 105(3): 430-437.
[17] Kiniwa Y, Miyahara Y, Wang H Y, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer [J]. Clinical Cancer Research, 2007, 13(23): 6947-6958.
CUSABIO team. Identify Treg Cells. You Can Do This.. https://www.cusabio.com/c-20978.html








Comments
Leave a Comment