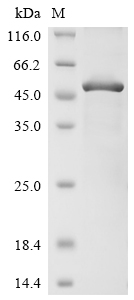
Recombinant Human Triggering receptor expressed on myeloid cells 2 (TREM2) is produced in E. coli and contains amino acids 19-174. This partial protein features an N-terminal 10xHis-GST tag along with a C-terminal Myc tag, which makes purification and detection more straightforward. The protein achieves purity greater than 85% based on SDS-PAGE analysis, suggesting it should work reliably in research settings.
TREM2 appears to be a receptor found mainly on myeloid cells. It seems to play an important role in immune system function, particularly in controlling inflammatory responses. Researchers studying neurodegenerative diseases and innate immunity have shown considerable interest in this receptor. Learning how TREM2 works and what regulates it may prove essential for scientists investigating its role in different cellular pathways and disease processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
TREM2 is an immunoglobulin domain-containing receptor requiring precise disulfide bond formation (e.g., C36-C110, C51-C60) and glycosylation (e.g., at N79) for native structure and function, as revealed by structural studies. E. coli, a prokaryotic system, lacks glycosylation machinery, and the large GST tag (∼26 kDa) may sterically hinder proper folding of the TREM2 extracellular domain (∼18 kDa). Thus, this protein likely lacks native structure and bioactivity for applications dependent on conformational integrity.
1. Antibody Development and Validation
This recombinant TREM2 fragment (19-174aa) can be used as an immunogen for generating antibodies targeting linear epitopes, but not conformational epitopes. The dual tags provide flexibility for purification and detection. However, antibodies raised against a misfolded protein may not recognize native TREM2 in physiological contexts (e.g., on microglia). For ELISA-based screening, the protein may work for initial tests, but specificity validation must include natively folded TREM2 controls.
2. Protein-Protein Interaction Studies
Pull-down assays using this tagged fragment are unreliable without folding verification. TREM2 ligand binding (e.g., to glycosaminoglycans or phospholipids) requires a precise surface topology, which is likely compromised by misfolding and the large GST tag. If misfolded, false-negative or non-specific interactions may dominate. Surface plasmon resonance should only be attempted if bioactivity is confirmed.
3. Structural and Biochemical Characterization
This protein is not suitable for structural studies without rigorous folding validation. Techniques like circular dichroism or dynamic light scattering would yield misleading data if the protein is misfolded. The tags and E. coli-derived non-glycosylated structure deviate significantly from native TREM2, whose crystal structure was determined using glycosylated, mammalian-expressed protein.
4. ELISA-Based Detection Assays
The protein can be used as a standard only for detecting tag-specific signals (e.g., anti-Myc or anti-GST antibodies), not for quantifying native TREM2. If misfolded, it will not accurately simulate endogenous TREM2 in sandwich ELISAs targeting conformational epitopes. Standard curves for TREM2 quantification require a bioactive reference material
Final Recommendation & Action Plan
Given the high likelihood of misfolding, prioritize validating the protein before use. Recommended actions: (1) employ circular dichroism spectroscopy to compare secondary structure with reference data from mammalian-expressed TREM2; (2) perform a ligand-binding assay using known interactors (e.g., sulfated glycans) to test bioactivity; and (3) use size-exclusion chromatography with multi-angle light scattering (SEC-MALS) to check for aggregation. If validation fails, repurpose the protein only for tag-based controls or linear epitope antibody development. For reliable TREM2 studies, switch to a mammalian expression system (e.g., CHO cells) that supports glycosylation and native folding.