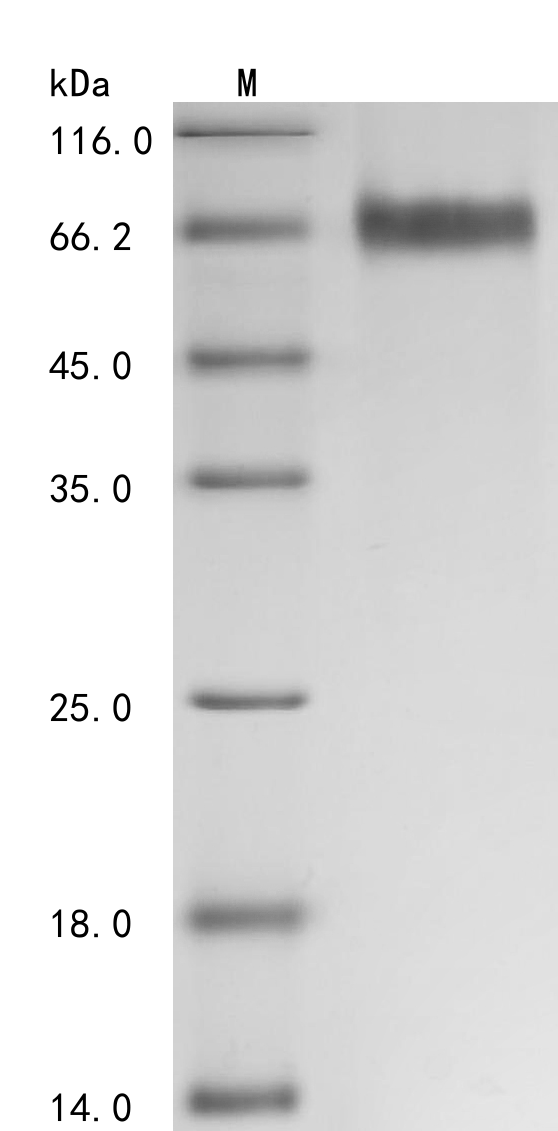
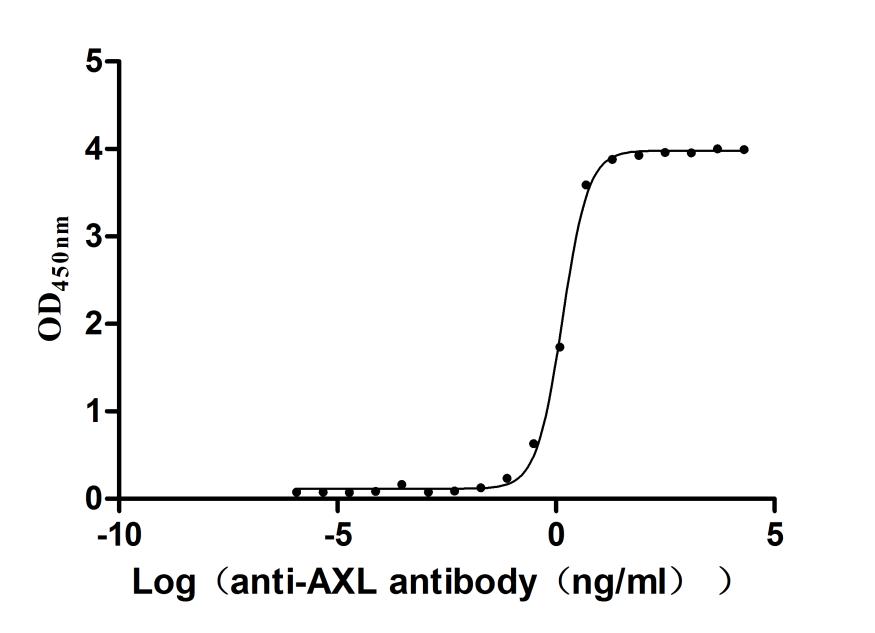
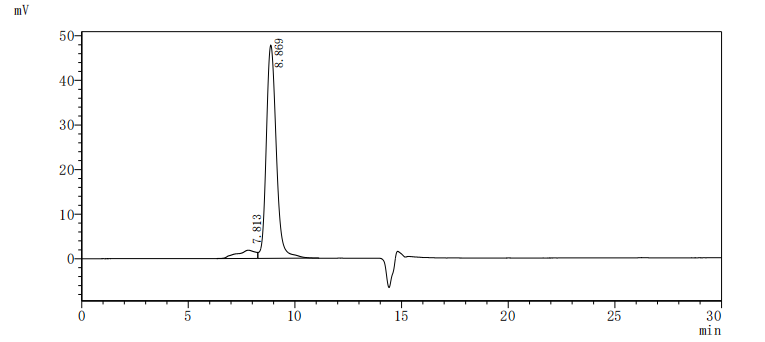
Producing the recombinant human tyrosine-protein kinase receptor UFO (AXL) involves a coherent set of steps: gene cloning, plasmid design, expression, purification, and analysis. The gene segment encoding 26-451aa of AXL is amplified using designed primers and inserted into a plasmid with a C-terminal 10xHis-tag. After transfection of mammalian cells with the recombinant plasmid, a selective antibiotic is applied 24 hours later to screen cells expressing the target AXL protein. The selected cells are cultured for protein expression. Following cell lysis, the AXL protein is purified using Ni-NTA affinity chromatography from the supernatant. SDS-PAGE confirms the recombinant AXL protein purity exceeds 95%. The endotoxin levels of the AXL protein are measured at <1.0 EU/μg via the LAL method. Functional ELISA validates this recombinant AXL protein binding to the AXL recombinant antibody (CSB-RA326981MA1HU), with an EC50 of 1.308-1.500 ng/mL.
Human AXL is a member of the TAM (Tyro3, AXL, Mer) family of receptor tyrosine kinases. AXL plays a pivotal role in various cellular processes, including cell survival, proliferation, migration, and angiogenesis. AXL is activated by its ligand, growth arrest-specific 6 (Gas6), which induces receptor dimerization and subsequent autophosphorylation, triggering downstream signaling pathways that can promote tumorigenesis and metastasis [4][6][10].
AXL is overexpressed in several cancer types, including breast, lung, and colorectal cancers. Its expression is often associated with poor prognosis and therapeutic resistance. Studies have shown that AXL mediates resistance to therapies targeting the EGFR in cancers such as head and neck squamous cell carcinoma and triple-negative breast cancer [1][2][7]. This resistance is partly due to AXL's ability to activate alternative signaling pathways that circumvent the inhibited EGFR, thereby allowing cancer cells to survive and proliferate despite treatment [11][13].
AXL has also been implicated in viral infections, notably as a receptor for the Zika virus [6][14]. The receptor's involvement in immune modulation and angiogenesis further underscores its importance in both cancer progression and potential therapeutic strategies [3][12]. Given its multifaceted roles, AXL is being explored as a therapeutic target, with strategies aimed at inhibiting its function showing promise in preclinical models [5][8][9].
References:
[1] Brand, T., Iida, M., Stein, A., Corrigan, K., Braverman, C., Luthar, N., … & Wheeler, D. (2014). Axl mediates resistance to cetuximab therapy. Cancer Research, 74(18), 5152-5164. https://doi.org/10.1158/0008-5472.can-14-0294
[2] Elkabets, M., Pazarentzos, E., Juric, D., Sheng, Q., Pelossof, R., Brook, S., … & Baselga, J. (2015). Axl mediates resistance to pi3kα inhibition by activating the egfr/pkc/mtor axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell, 27(4), 533-546. https://doi.org/10.1016/j.ccell.2015.03.010
[3] Han, Y., Li, G., Zhang, Z., Zhang, X., Zhao, B., & Yang, H. (2023). Axl promotes intracranial aneurysm rupture by regulating macrophage polarization toward m1 via stat1/hif-1α. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1158758
[4] Jin, G., Wang, Z., Wang, J., Zhang, L., Chen, Y., Yuan, P., … & Liu, D. (2016). Expression of axl and its prognostic significance in human breast cancer. Oncology Letters, 13(2), 621-628. https://doi.org/10.3892/ol.2016.5524
[5] Kariolis, M., Miao, Y., Jones, D., Kapur, S., Mathews, I., Giaccia, A., … & Cochran, J. (2014). An engineered axl 'decoy receptor' effectively silences the gas6-axl signaling axis. Nature Chemical Biology, 10(11), 977-983. https://doi.org/10.1038/nchembio.1636
[6] Li, S., DeLalio, L., Isakson, B., & Wang, T. (2016). Axl-mediated productive infection of human endothelial cells by zika virus. Circulation Research, 119(11), 1183-1189. https://doi.org/10.1161/circresaha.116.309866
[7] Meyer, A., Miller, M., Gertler, F., & Lauffenburger, D. (2013). The receptor axl diversifies egfr signaling and limits the response to egfr-targeted inhibitors in triple-negative breast cancer cells. Science Signaling, 6(287). https://doi.org/10.1126/scisignal.2004155
[8] Onken, J., Torka, R., Korsing, S., Radke, J., Krementeskaia, I., Nieminen, M., … & Vajkoczy, P. (2016). Inhibiting receptor tyrosine kinase axl with small molecule inhibitor bms-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget, 7(9), 9876-9889. https://doi.org/10.18632/oncotarget.7130
[9] Scaltriti, M., Elkabets, M., & Baselga, J. (2016). Molecular pathways: axl, a membrane receptor mediator of resistance to therapy. Clinical Cancer Research, 22(6), 1313-1317. https://doi.org/10.1158/1078-0432.ccr-15-1458
[10] Scherschinski, L., Prem, M., Kremenetskaia, I., Tinhofer, I., Vajkoczy, P., Karbe, A., … & Onken, J. (2022). Regulation of the receptor tyrosine kinase axl in response to therapy and its role in therapy resistance in glioblastoma. International Journal of Molecular Sciences, 23(2), 982. https://doi.org/10.3390/ijms23020982
[11] Su, C., Hsu, T., Sung, S., Huang, M., Chen, K., Huang, C., … & Liao, P. (2021). axl is crucial for e1a‐enhanced therapeutic efficiency of egfr tyrosine kinase inhibitors through nfi in breast cancer. Environmental Toxicology, 36(7), 1278-1287. https://doi.org/10.1002/tox.23125
[12] Tanaka, M. and Siemann, D. (2019). Axl signaling is an important mediator of tumor angiogenesis. Oncotarget, 10(30), 2887-2898. https://doi.org/10.18632/oncotarget.26882
[13] Tsukita, Y., Fujino, N., Miyauchi, E., Saito, R., Fujishima, F., Itakura, K., … & Ichinose, M. (2019). Axl kinase drives immune checkpoint and chemokine signalling pathways in lung adenocarcinomas. Molecular Cancer, 18(1). https://doi.org/10.1186/s12943-019-0953-y
[14] Zwernik, S., Adams, B., Raymond, D., Kassam, A., Rovin, R., & Akhtar, P. (2021). Axl receptor is required for zika virus strain mr-766 infection in human glioblastoma cell lines. Molecular Therapy — Oncolytics, 23, 447-457. https://doi.org/10.1016/j.omto.2021.11.001