[1] Albanes, Demetrius, et al. "The blood proteome of imminent lung cancer diagnosis." Nature Communications 14.1 (2023).
[2] Wortmann, Andreas, et al. "The cell surface glycoprotein CDCP1 in cancer-insights, opportunities, and challenges." IUBMB life 61.7 ( 2009): 723-730.
[3] Murakami, Yuichi, et al. "AXL/CDCP1/SRC axis confers acquired resistance to osimertinib in lung cancer." Scientific Reports 12.1 (2022): 8983.
[4] Uekita, Takamasa, and Ryuichi Sakai. "Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis." Cancer science 102.11 (2011): 1943-1948.
[5] Qi, Xiao, et al. "CDCP1: A promising diagnostic biomarker and therapeutic target for human cancer." Life Sciences 301 (2022): 120600.
[6] Hooper, John D., et al. "Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen." Oncogene 22.12 (2003): 1783-1794.
[7] Casar, B., et al. "In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated β1 integrin and induction of FAK/PI3K/Akt motility signaling." Oncogene 33.2 (2014): 255-268.
[8] Alajati, Abdullah, et al. "CDCP1 overexpression drives prostate cancer progression and can be targeted in vivo." the Journal of clinical investigation 130.5 (2020): 2435-2450.
[9] Pollan, Sara G., et al. "Regulation of inside-out β1-integrin activation by CDCP1." Oncogene 37.21 (2018): 2817-2836.
[10] He, Yaowu, et al. "CDCP1 enhances Wnt signaling in colorectal cancer promoting nuclear localization of β-catenin and E-cadherin." Oncogene 39.1 (2020) : 219-233.
[11] Forte, Luca, et al. "The PDGFRβ/ERK1/2 pathway regulates CDCP1 expression in triple-negative breast cancer." BMC cancer 18 (2018): 1-11.
[12] Chiu, Kuo-Liang, et al. "ADAM9 enhances CDCP1 by inhibiting miR-1 through EGFR signaling activation in lung cancer metastasis." Oncotarget 8.29 (2017). : 47365.
[13] Emerling, Brooke M., et al. "Identification of CDCP1 as a hypoxia-inducible factor 2α (HIF-2α) target gene that is associated with survival in clear cell renal cell carcinoma patients." Proceedings of the National Academy of Sciences 110.9 (2013): 3483-3488.
[14] Chandrasekaran, Balaji, et al. "Antiandrogen-Equipped Histone Deacetylase Inhibitors Selectively Inhibit Androgen Receptor (AR) and AR-Splice Variant (AR-SV) in Castration-Resistant Prostate Cancer (CRPC)." Cancers 15.6 (2023): 1769.
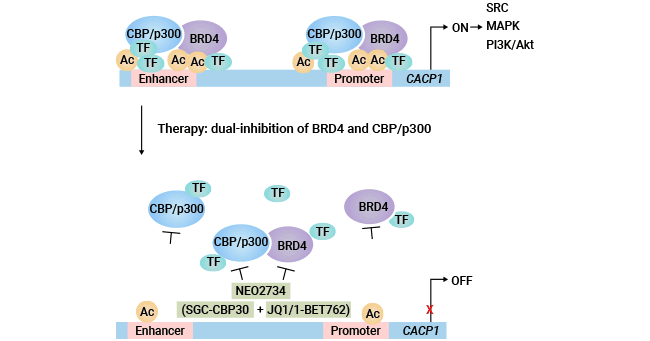
[15] Ji, Donglei, et al. "Targeting CDCP1 gene transcription coactivated by BRD4 and CBP/p300 in castration-resistant prostate cancer." Oncogene 41.23 ( 2022): 3251-3262.
[16] Alajati, A., J. Chen, and A. Alimonti. "CDCP1 initiates tumorigenesis and cooperates with PTEN loss to promote senescence evasion and prostate cancer progression." Annals of Oncology 28 (2017): v1.
[17] Chou, Chiang-Ting, et al. "Prognostic significance of CDCP1 expression in colorectal cancer and effect of its inhibition on invasion and migration." Annals of surgical oncology 22 (2015): 4335-4343.
[18] Karachaliou, Niki, et al. "Common co-activation of AXL and CDCP1 in EGFR-mutation-positive non-small cell lung cancer associated with poor prognosis. " EBioMedicine 29 (2018): 112-127.
[19] Jiang, Tao, et al. "Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta- analysis." Cancer Medicine 5.6 (2016): 1055-1065.
[20] Chiu, Kuo-Liang, et al. "ADAM9 enhances CDCP1 protein expression by suppressing miR-218 for lung tumor metastasis." scientific reports 5.1 (2015). 16426.
[21] Kuhara, Keisuke, et al. "CUB domain-containing protein 1 (CDCP1) is down-regulated by active hexose-correlated compound in human pancreatic cancer cells." Anticancer Research 38.11 (2018): 6107-6111.
[22] Alajati, Abdullah, et al. "Interaction of CDCP1 with HER2 enhances HER2-driven tumorigenesis and promotes trastuzumab resistance in breast cancer." Cell reports 11.4 (2015): 564-576.
[23] Cui, Yan-Hong, et al. "FBXL14 abolishes breast cancer progression by targeting CDCP1 for proteasomal degradation." Oncogene 37.43 (2018): 5794-5809.
[24] Shen, N., et al. "Effect of NLK on the proliferation and invasion of laryngeal carcinoma cells by regulating CDCP1." European Review for Medical & Pharmacological Sciences 23.14 (2019).
[25] Huang, Lijun, et al. "CUB domain-containing protein-1 promotes proliferation, migration and invasion in cervical cancer cells." Cancer Management and Research (2020): 3759-3769.
[26] Cao, Manqing, et al. "HIF-2α regulates CDCP1 to promote PKCδ-mediated migration in hepatocellular carcinoma." Tumor Biology 37 (2016): 1651-1662.
[27] Gioia, Romain, et al. "Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells." Blood, The Journal of the American Society of Hematology 118.8 (2011): 2211-2221.
[28] Heitmann, Jonas S., et al. "Identification of CD318 (CDCP1) as novel prognostic marker in AML." Annals of Hematology 99 (2020): 477-486.
[29] Ebian, Huda F., et al. "Evaluation of CDCP1 (CD318) and endoglin (CD105) expression as prognostic markers in acute myeloid leukemia." Cancer Biomarkers 34.2 (2022): 285-296.

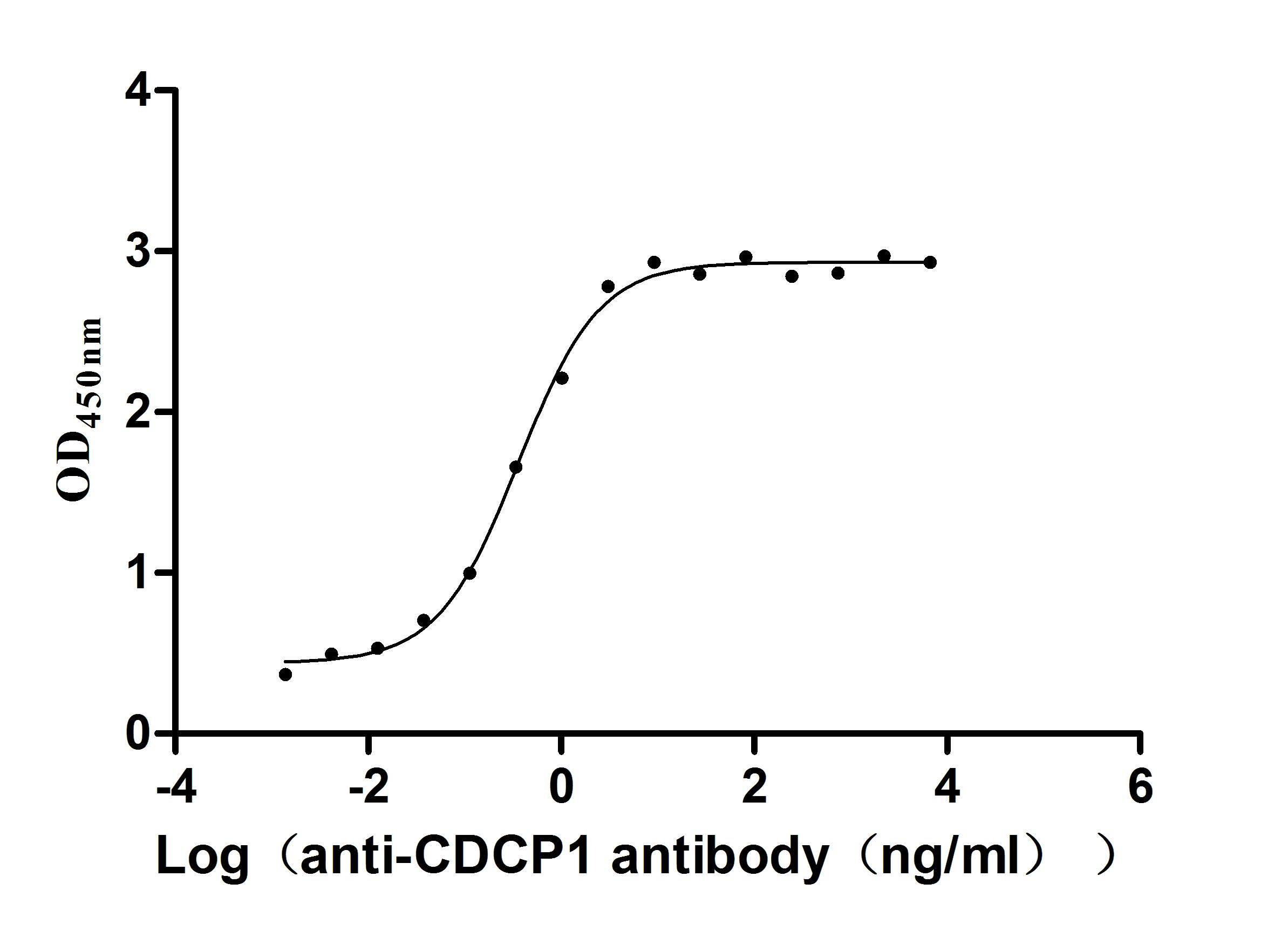
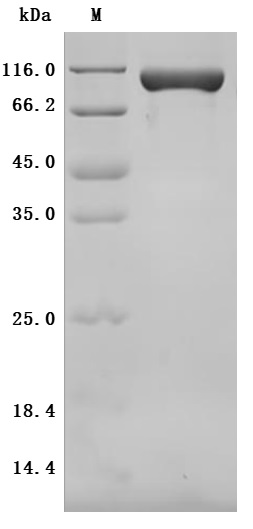
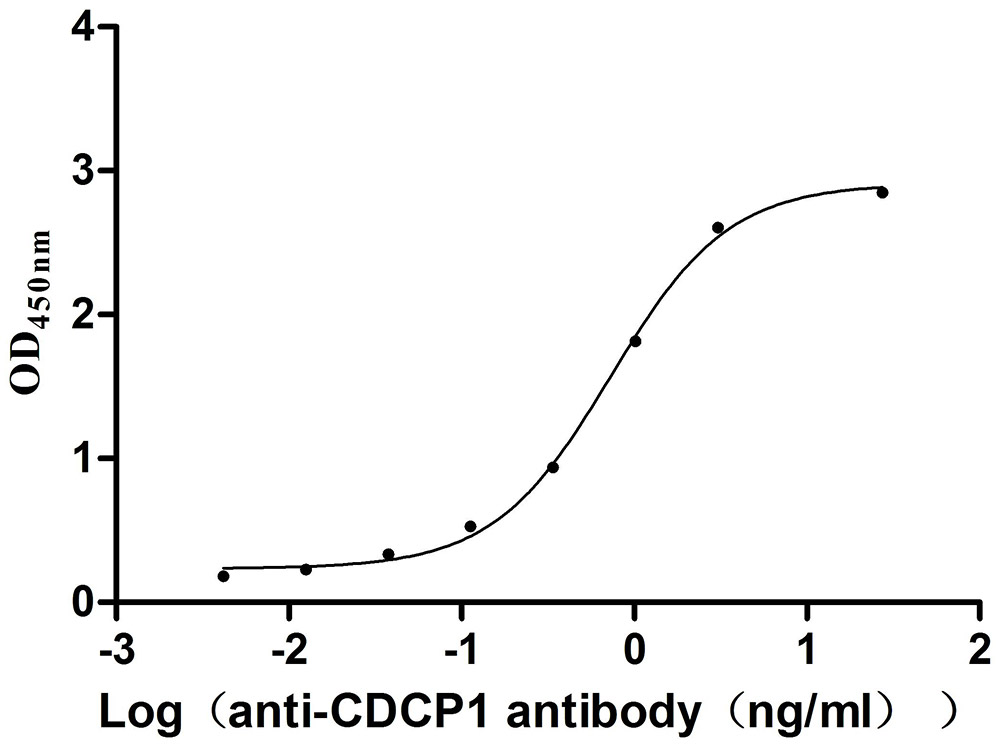
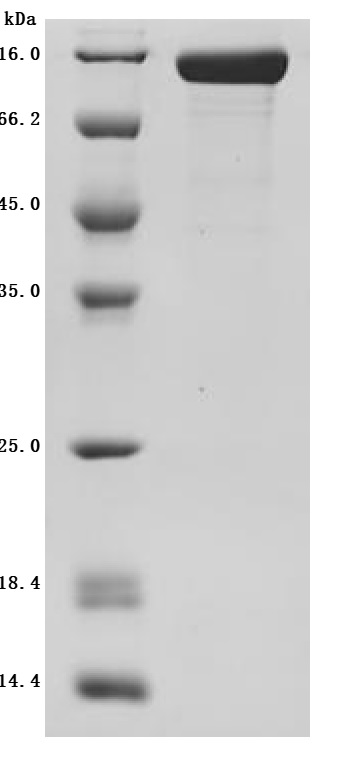



Comments
Leave a Comment