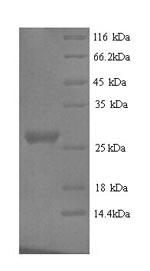
Recombinant Mouse Nidogen-1 (Nid1) comes from yeast expression and covers a partial sequence spanning amino acids 428 to 665. It includes an N-terminal 6xHis-tag that makes purification straightforward. The protein shows a purity level exceeding 90% when verified by SDS-PAGE, which appears to ensure high-quality results in research applications. This product is intended for research use only.
Nidogen-1 serves as an essential component of the extracellular matrix. It's primarily involved in the structural organization and stability of basement membranes. The protein plays a critical role in cell-matrix interactions and participates in various biological pathways, including tissue development and repair processes. Its importance in research likely stems from its involvement in cell adhesion and signaling, making it a key focus in studies related to tissue engineering and regenerative medicine.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, recombinant mouse Nid1 is produced in a yeast expression system as a partial fragment (428-665aa) with an N-terminal 6xHis-tag. Nid1 is an extracellular matrix glycoprotein that requires precise folding, disulfide bond formation, and proper tertiary structure for its biological function in basement membrane assembly. Yeast expression systems provide eukaryotic folding machinery capable of supporting disulfide bond formation and some post-translational modifications, which increases the probability of correct folding compared to prokaryotic systems. However, the partial nature of the fragment (238aa, representing approximately 25% of full-length Nid1) may not fold correctly independently, as its structural context depends on the full protein architecture. The N-terminal His-tag may interfere with the native structure. No validation data (e.g., ligand binding assays, circular dichroism) are provided. Therefore, while yeast expression offers advantages, the protein's folding status and bioactivity cannot be confirmed without experimental validation.
1. Extracellular Matrix Interaction Studies
If the recombinant Nid1 fragment is correctly folded, it could be used to study interactions with ECM components like laminin or collagen IV, as the His-tag facilitates immobilization for binding assays. However, if misfolded, interaction domains may be altered, leading to non-specific binding or failure to recognize genuine biological partners, compromising the validity of identified interaction networks. The partial nature may also lack key binding sites present in full-length Nid1.
2. Antibody Development and Validation
This application is suitable as antibody generation primarily relies on linear epitope recognition, which is independent of folding status. The defined fragment provides specific epitopes for antibody production. However, if misfolded, generated antibodies may not optimally recognize conformation-dependent epitopes of native, full-length Nid1 in biological contexts, limiting utility for functional studies.
3. Protein Structure-Function Analysis
If properly folded, the fragment could be used for limited structural studies of this specific domain. However, if misfolded, structural data would misrepresent the native protein's architecture, and the partial nature limits insights into full-length Nid1 behavior. The His-tag may also interfere with detailed structural analysis.
4. Cell Adhesion and Migration Assays
If correctly folded and functional, the recombinant fragment could serve as a substrate for cell adhesion studies. However, if misfolded, it may not support proper cell adhesion, leading to false-negative results. The partial nature may lack critical domains required for full biological activity in cell-matrix interactions.
Final Recommendation & Action Plan
Before employing this recombinant Nid1 fragment in any application, it is essential to validate its folding and bioactivity through biophysical methods (e.g., circular dichroism for secondary structure, size-exclusion chromatography for oligomeric state) and functional assays (e.g., binding tests with known ligands like laminin or collagen IV); if validation confirms proper folding and function, proceed with applications while noting the limitations of the partial fragment; if misfolded, consider using full-length Nid1 expressed in mammalian systems for better physiological relevance or obtain a commercially validated standard; for immediate use, antibody development can proceed with the understanding that antibodies may require additional validation against native protein; avoid functional studies until proper folding is confirmed. Always include appropriate controls (e.g., full-length Nid1, known ligands) in experiments to ensure reliability.