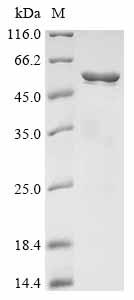
This recombinant Streptococcus agalactiae Group B streptococcal surface immunogenic protein (sip) is expressed in E. coli, covering the full-length mature protein from amino acids 26 to 434. It includes an N-terminal 10xHis tag and a C-terminal Myc tag, which helps with purification and detection. The product achieves purity greater than 85%, as verified by SDS-PAGE, making it suitable for various research applications.
The surface immunogenic protein (sip) from Streptococcus agalactiae appears to play a crucial role in how the bacterium interacts with host immune systems. Its location on the bacterial surface likely makes it an important target in studies focusing on bacterial pathogenesis and vaccine development. This protein has drawn significant interest from researchers trying to understand the mechanisms behind bacterial infection and immune response.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Since Sip is a bacterial protein, the E. coli expression system has a higher probability of producing a correctly folded protein compared to eukaryotic proteins, as it shares a similar cellular environment and lacks complex post-translational modifications. The protein contains the full-length mature region (26-434aa), which is the functionally relevant domain. However, the activity is explicitly stated as unknown and unvalidated. Therefore, while the probability of correct folding is reasonably high, it cannot be guaranteed without experimental confirmation. The presence of two tags (N-terminal His, C-terminal Myc) could potentially, though not necessarily, interfere with the native structure or function.
1. Antibody Development and Characterization
This application is generally suitable. The recombinant sip protein can effectively serve as an immunogen for generating antibodies. Even if the protein is not in its native conformation, it can still elicit antibodies that recognize linear epitopes, which are useful for techniques like Western blotting. The dual tags facilitate purification and screening. The >85% purity is adequate for immunization. It should be noted that antibodies generated against a potentially misfolded protein might not recognize the native protein on the bacterial surface in its natural context. Validation against native Sip is recommended.
2. Protein-Protein Interaction Studies
This application is highly dependent on correct protein folding. The tags are convenient for immobilization in pull-down assays. However, if the protein is misfolded, its ability to interact with true biological binding partners (e.g., host cell receptors) will be compromised, leading to false-negative or non-physiological results. This application should be considered high-risk without prior folding/activity validation.
3. Immunological Assay Development
This application is appropriate and is one of the most reliable uses for this reagent. For developing research immunoassays like ELISA or Western blot, the correct tertiary structure is less critical than for functional studies. The protein serves as a well-defined, pure antigen for standardizing detection methods. The tags are highly beneficial for quantification and quality control.
4. Biochemical Characterization Studies
This application is not only appropriate but is actually a prerequisite for others. The described studies (stability, folding, pH tolerance) are the very methods needed to determine the protein's state. These biophysical assays can directly assess whether the protein is folded, monomeric, and stable. The results from these studies should inform the feasibility of the more demanding applications, like protein-protein interaction studies.
Final Recommendation & Action Plan
Given the high likelihood but unconfirmed status of correct folding, the recommended course of action is to prioritize biochemical and biophysical characterization (Application #4) to assess protein folding and stability before proceeding to functional studies. If the protein demonstrates a monodisperse state and expected secondary structure, it can then be cautiously used for interaction studies (#2), with the understanding that results require confirmation using alternative methods. Applications for antibody development (#1) and immunoassay development (#3) are lower risk and can proceed concurrently, as they are less dependent on native conformation. Ultimately, correlating findings with experiments using native protein or genetic methods is crucial for validating any biological insights.