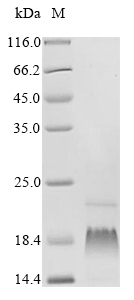
Recombinant Human Gamma-secretase subunit PEN-2 (PSENEN) is produced through an in vitro E. coli expression system. The full-length protein spans amino acids 1-101 and comes equipped with an N-terminal 10xHis-tag that makes purification and detection more straightforward. SDS-PAGE analysis shows the product achieves greater than 85% purity, which appears sufficient for most research applications demanding high-quality proteins.
Gamma-secretase subunit PEN-2 represents an essential piece of the gamma-secretase complex puzzle. This complex drives intramembrane proteolysis—a process that's proving more intricate than researchers initially thought. The protein appears to regulate both Notch signaling and amyloid precursor protein processing, though the exact mechanisms may be more nuanced than current models suggest. PEN-2's involvement in these pathways has made it a prime target for scientists studying cellular signaling and neurodegenerative diseases.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
PSENEN requires integration into a lipid membrane and association with other subunits (Presenilin, Nicastrin, APH-1) to achieve its native structure and function. The bacterial expression system lacks the necessary membrane environment and eukaryotic chaperones, making it highly unlikely that this recombinant protein will adopt its correct transmembrane topology or functional conformation. While the protein may be soluble, it is almost certainly misfolded and inactive as a gamma-secretase component. A misfolded, soluble version of a membrane protein like PSENEN is prone to expose hydrophobic regions that cause non-specific (false-positive) binding. It will not be integrated into a membrane and thus cannot interact with its physiological partners in the correct orientation or context, leading to false negatives. Any interaction data would be uninterpretable.
1. Antibody Development and Validation
This recombinant PSENEN can serve as a suitable immunogen for generating antibodies that recognize linear epitopes of the protein. The full-length sequence ensures coverage of the entire amino acid sequence. These antibodies will be useful for techniques like Western blotting under denaturing conditions. However, they may not efficiently recognize the native, membrane-embedded PSENEN within the gamma-secretase complex in cellular contexts (e.g., for immunofluorescence or native immunoprecipitation).
2. Biochemical Characterization and Stability Studies
This is the essential first step to characterize the biophysical properties of this specific protein preparation. Techniques like SEC-MALS (Size Exclusion Chromatography with Multi-Angle Light Scattering) and CD (Circular Dichroism) can determine its oligomeric state, stability, and secondary structure in solution. This data provides crucial quality control data for the protein itself, not the native membrane protein.
Final Recommendation & Action Plan
The expression system is fundamentally incapable of producing a correctly folded, functional version of this transmembrane protein, severely limiting its applications. The immediate and only reliable step is Application 2 (Biochemical Characterization) to understand the physical properties of the produced soluble protein. It can be used for Application 1 (Antibody Development against linear epitopes). Interaction studies and complex reconstitution must be avoided entirely as they require the native structure. For functional studies of PSENEN, the only valid path is to study the endogenous protein from mammalian cells or co-express the subunits in a eukaryotic membrane-containing system (e.g., insect cell membranes, proteoliposomes) that supports correct folding and complex assembly. This protein should be viewed strictly as a source of antigen for antibody production, not as a functional protein.