CAR-T cell therapy has shown remarkable efficacy in treating hematologic malignancies but faces challenges in solid tumors due to the immunosuppressive tumor microenvironment, which limits CAR-T cell proliferation, persistence, and cytotoxicity. GITR and its ligand GITRL, as novel costimulatory molecules, enhance T cell survival, proliferation, and differentiation while suppressing regulatory T cell activity, thus improving CAR-T cell therapy in solid tumors.
A recent study by Du Bing's team at East China Normal University demonstrated that CAR-T cells overexpressing GITRL significantly enhanced solid tumor treatment efficacy, offering new strategies for CAR-T therapy in solid tumors. This research was published in Molecular Therapy on January 24, 2025 [1].
TNFSF18 (tumor necrosis factor superfamily member 18), also known as GITRL, is located on human chromosome 1p36.13 and is primarily expressed on antigen-presenting cells. It plays a crucial role in immune regulation, tumor development, and various diseases through its interaction with GITR, modulating T cell activation, proliferation, and survival.
1. Mechanism of Action
TNFSF18, mainly expressed on antigen-presenting cells, binds to GITR to regulate T cell functions, making it an immune checkpoint gene [2]. In the tumor microenvironment, TNFSF18-GITR signaling promotes tumor immune evasion, growth, and metastasis by modulating interactions between tumor and immune cells, and also drives tumor angiogenesis.
2. Related Signaling Pathways
TNFSF18 is involved in key signaling pathways such as NF-κB, MAPK, and PI3K/AKT. It activates NF-κB to boost inflammatory and immune gene expression, MAPK to regulate cell proliferation and survival, and PI3K/AKT to enhance cell survival and anti-apoptosis, with abnormal activation linked to multiple tumors [3].
3. Associated Diseases
3.1 Malignant Pleural Mesothelioma (MPM)
TNFSF18 overexpression in MPM correlates with tumor progression and poor prognosis. It drives tumor cell proliferation, migration, and invasion, and modulates the tumor microenvironment. Targeting TNFSF18-GITR signaling may overcome drug resistance and improve treatment outcomes [4].
3.2 Non-Small Cell Lung Cancer (NSCLC)
In NSCLC, TNFSF18 expression predicts patient prognosis. It promotes tumor cell proliferation, survival, invasion, and metastasis via downstream pathways and affects the tumor microenvironment, suggesting immunotherapeutic strategies targeting TNFSF18 could enhance treatment efficacy [5].
3.3 Autoimmune Diseases
TNFSF18 plays a role in autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus. In rheumatoid arthritis, it drives synovial cell proliferation and inflammation, while in systemic lupus erythematosus, it promotes autoreactive lymphocyte activation and autoantibody production [6].
3.4 Other Diseases
TNFSF18 is also implicated in inflammatory bowel disease and multiple sclerosis, where it influences intestinal inflammation and central nervous system immune regulation, respectively [7].
4. Drug Development
Drug development targeting TNFSF18 focuses on inhibitors, modulators, and monoclonal antibodies. Anti-TNFSF18 antibodies block TNFSF18-GITR signaling, inhibiting tumor growth and enhancing antitumor immunity. Small molecule inhibitors also show potential as anticancer agents, while Du Bing's research highlights TNFSF18's role in CAR-T cell therapy.
5. Product Recommendations
TNFSF18's role in immune regulation and disease progression makes it a valuable research target. CUSABIO offers high-quality TNFSF18 products, including proteins, antibodies, and ELISA kits, to support research into TNFSF18's mechanisms and clinical potential.
● TNFSF18/GITRL Antibody
TNFSF18 Antibody (CSB-PA891791ESR1HU)
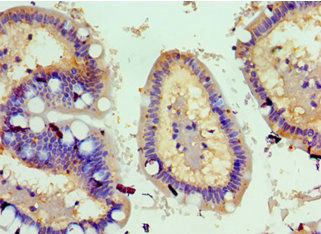
Immunohistochemistry of paraffin-embedded human small intestine tissue using CSB-PA891791ESR1HU at dilution of 1:100
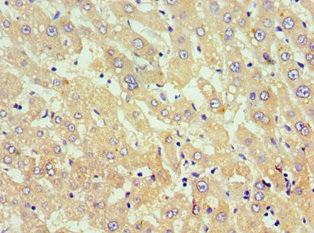
Immunohistochemistry of paraffin-embedded human liver tissue using CSB-PA891791ESR1HU at dilution of 1:100
References
[1] Qin J;Du B, et al. GITRL enhances cytotoxicity and persistence of CAR-T cells in cancer therapy. Mol Ther. 2025.
[2] Kim K, Song JE, Joo JB, et al. Genome-wide association study of mammary gland tumors in Maltese dogs. Front Vet Sci. 2023;10:1255981.
[3] Hervás-Corpión I, Navarro-Calvo J, Martín-Climent P, et al. Defining a correlative transcriptional signature associated with bulk histone H3 acetylation levels in adult glioblastomas. Cells. 2023;12(3):374.
[4] Wu L, Dell'anno I, Lapidot M, et al. Progress of malignant mesothelioma research in basic science: A review of the 14th international conference of the international mesothelioma interest group (iMig2018). Lung Cancer. 2019;138:138-145.
[5] Chen B, Yang M, Xu Y, et al. Immune-related genes and gene sets for predicting the response to anti-programmed death 1 therapy in patients with primary or metastatic non-small cell lung cancer. Oncol Lett. 2021;22(2):540.
[6] Brandstetter G, Vladimer G, Superti-Furga G, et al. FOXO3 is involved in the tumor necrosis factor-driven inflammatory response in fibroblast-like synoviocytes. Lab Invest. 2019;99(1):130-142.
[7] Liu B, Kern P, Wu Y, et al. Glucocorticoid-induced TNF receptor family-related protein ligand promotes the development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(7):1305-1314.
CUSABIO team. TNFSF18: Unlocking New Frontiers in Cancer Immunotherapy. https://www.cusabio.com/c-21214.html

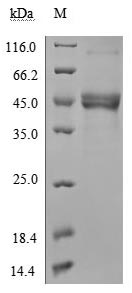
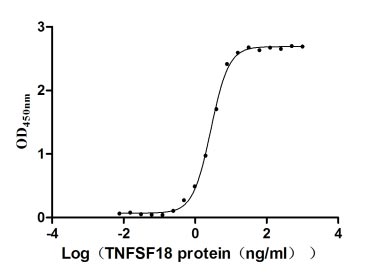




Comments
Leave a Comment