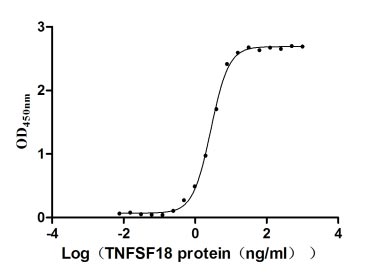
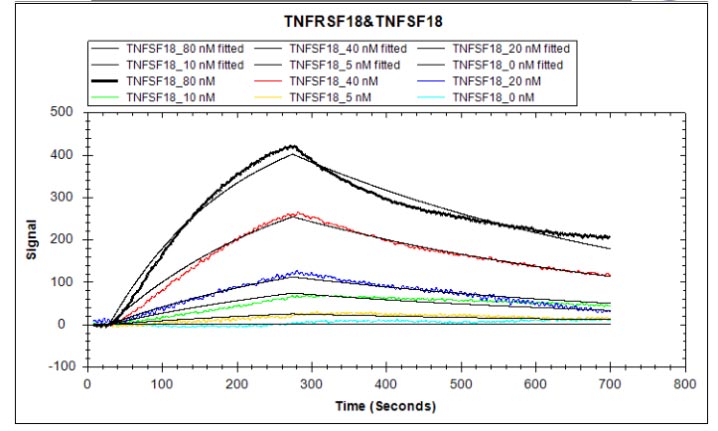
This N-terminal hFc-Flag-tagged recombinant human TNFSF18 protein (amino acids 72-199) is produced in mammalian cells, ensuring proper post-translational modifications. With >90% purity (SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method), this recombinant TNFSF18 protein is suitable for sensitive immunological studies. Functional assays confirm its bioactivity. In ELISA, it binds immobilized TNFRSF18 with the EC50 of 2.565–2.940 ng/mL, while LSPR analysis reveals a binding affinity constant of 38.5 nM to hFc-tagged human TNFRSF18 (CSB-MP896537HU). Its dual hFc-Flag tag facilitates purification and detection. Provided as lyophilized powder, this recombinant TNFSF18 protein is optimized for stability and reconstitution, serving as a critical tool for investigating TNFSF18-TNFRSF18 interactions in immune regulation, inflammatory responses, and therapeutic antibody development.
The human TNFSF18 protein, also known as GITRL, is a member of the TNF superfamily and plays a crucial role in modulating immune responses. It is primarily expressed on various immune cells, including activated T cells and regulatory T cells (Tregs), where it functions in co-stimulatory signaling pathways essential for T cell activation and differentiation [1][2]. Its receptor, TNFRSF18 (commonly referred to as GITR), is found on naive T cells, activated T cells, and Tregs, allowing for interactions crucial for maintaining immune homeostasis and balancing immune activation and suppression [3][4].
One significant function of TNFSF18 is its ability to modulate Treg activity. Evidence suggests that engagement of TNFRSF18 by its ligand TNFSF18 can diminish the suppressive functions of Tregs, thus enhancing effector T cell activity and promoting a more robust immune response [2][5]. This characteristic is particularly noteworthy in the context of cancer, where high levels of TNFSF18 expression correlate with improved anti-tumor responses in various animal models [6].
In addition to its role in cancer immune responses, TNFSF18 is implicated in various inflammatory conditions. It has been shown to participate in the immune responses associated with conditions like atherosclerosis, where its action can influence the activation and recruitment of immune cells [7]. Furthermore, the overexpression of TNFSF18 has been linked to certain autoimmune diseases, emphasizing the need for tightly regulated GITR-TNFSF18 interactions to maintain immune balance and prevent pathological immune activation [8]. Its dual role that enhances effector T cell function while simultaneously tempering the suppressive activities of Tregs highlights its potential as a therapeutic target in the treatment of cancer and autoimmune diseases, providing pathways for innovative immunomodulatory therapies [8][9].
References:
[1] B. Chen, M. Yang, K. Li, J. Li, L. Xu, F. Xuet al., Immune‑related genes and gene sets for predicting the response to anti‑programmed death 1 therapy in patients with primary or metastatic non‑small cell lung cancer, Oncology Letters, vol. 22, no. 1, 2021. https://doi.org/10.3892/ol.2021.12801
[2] T. Placke, H. Kopp, & H. Salih, Glucocorticoid‐induced tnfr‐related (gitr) protein and its ligand in antitumor immunity: functional role and therapeutic modulation, Journal of Immunology Research, vol. 2010, no. 1, 2010. https://doi.org/10.1155/2010/239083
[3] A. Liu, F. Li, B. Wang, L. Yang, H. Xing, C. Suet al., Prognostic and immunological significance of calcium-related gene signatures in renal clear cell carcinoma, Frontiers in Pharmacology, vol. 13, 2022. https://doi.org/10.3389/fphar.2022.1055841
[4] D. Richards, V. Marschall, K. Billian-Frey, K. Heinonen, C. Merz, M. Mülleret al., Hera-gitrl activates t cells and promotes anti-tumor efficacy independent of fcγr-binding functionality, Journal for Immunotherapy of Cancer, vol. 7, no. 1, 2019. https://doi.org/10.1186/s40425-019-0671-4
[5] H. Ji, G. Liao, W. Faubion, A. Abadía‐Molina, C. Cozzo, F. Larouxet al., Cutting edge: the natural ligand for glucocorticoid-induced tnf receptor-related protein abrogates regulatory t cell suppression, The Journal of Immunology, vol. 172, no. 10, p. 5823-5827, 2004. https://doi.org/10.4049/jimmunol.172.10.5823
[6] H. Liu, W. Wu, G. Sun, T. Chia, L. Cao, X. Liuet al., Optimal target saturation of ligand-blocking anti-gitr antibody ibi37g5 dictates fcγr-independent gitr agonism and antitumor activity, Cell Reports Medicine, vol. 3, no. 6, p. 100660, 2022. https://doi.org/10.1016/j.xcrm.2022.100660
[7] J. Gao, S. Wang, & S. Liu, The involvement of protein tnfsf18 in promoting p‐stat1 phosphorylation to induce coronary microcirculation disturbance in atherosclerotic mouse model, Drug Development Research, vol. 82, no. 1, p. 115-122, 2020. https://doi.org/10.1002/ddr.21735
[8] G. Liao, M. O’Keeffe, G. Wang, B. Driel, R. Malefyt, H. Reineckeret al., Glucocorticoid-induced tnf receptor family-related protein ligand is requisite for optimal functioning of regulatory cd4+ t cells, Frontiers in Immunology, vol. 5, 2014. https://doi.org/10.3389/fimmu.2014.00035
[9] L. Li, Y. Li, J. Lin, & W. Pang, A pyroptosis-related gene signature predicts prognosis and tumor immune microenvironment in colorectal cancer, Technology in Cancer Research & Treatment, vol. 23, 2024. https://doi.org/10.1177/15330338241277584