The global tumor immunotherapy market is expected to exceed $200 billion by 2025, and NCR3, as a key "switch" for NK cell activation, has become a highly sought-after target for pharmaceutical companies. Multinational drug firms like Roche and Merck are exploring modulation through PROTAC technology, while domestic companies such as WuXi AppTec and Sansure Biotech are accelerating the development of recombinant proteins. NCR3 is transitioning from the lab to commercialization, sparking a wave of research and development.
1. Structural Characteristics of NCR3
Natural Cytotoxicity Triggering Receptor 3 (NCR3), also referred to as NKp30, is encoded by a gene located in the human Major Histocompatibility Complex (MHC) class III region [1] . NCR3 is a type I transmembrane receptor belonging to the immunoglobulin superfamily (IgSF) and consists of 190 amino acids, with a molecular weight of approximately 30kDa. Structurally, NCR3 includes a 138-amino acid extracellular immunoglobulin-like domain, which is formed by two antiparallel β-strands linked by disulfide bonds. This domain contains two potential N-linked glycosylation sites, crucial for ligand binding [2] . The receptor also features a 19-amino acid transmembrane domain, with a positively charged arginine residue that interacts with the immunoreceptor tyrosine-based activation motif (ITAM)-linking molecules CD3ζ and FcεRIγ, common features in other NK cell activation receptors. Additionally, NCR3 has a 33-amino acid cytoplasmic tail region, which lacks the typical ITAM consensus sequence [1] .
2. Mechanism of Action of NCR3
2.1 Activation of NK Cell Cytotoxicity
NCR3 is primarily expressed on the cell surface of mature NK cells and acts as a crucial activating receptor. When NCR3 binds to various non-MHC ligands secreted or expressed by tumor or virus-infected cells—such as heparan sulfate glycosaminoglycans (HS GAGs) and B7-H6—an intracellular signaling cascade is triggered[2]. Upon ligand binding, NCR3 interacts through its transmembrane domain with ITAM-linking molecules like CD3ζ and FcεRIγ, recruiting and activating spleen tyrosine kinase (Syk) and ζ-chain-associated protein kinase 70 (Zap70). Activated Syk and Zap70 phosphorylate transmembrane linking molecules, such as T cell activation linker (LAT) and non-T cell activation linker (NTAL), which subsequently activate downstream molecules like phosphoinositide 3-kinase (PI3K) and phospholipase C (PLC). These signaling events ultimately trigger calcium influx, leading to cytoskeletal rearrangement, cytotoxic granule release, and cytokine secretion, thereby enabling NK cells to kill target cells, directly lysing tumor or virus-infected cells [3].
2.2 Regulation of Cytokine Secretion
In addition to mediating direct cytotoxicity, NCR3 activation also regulates NK cell cytokine secretion. After NCR3 binds its ligands and activates downstream signaling pathways, NK cells release various cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) [4]. IFN-γ has broad immune-modulatory effects, enhancing macrophage phagocytosis and antigen presentation, promoting Th1 cell differentiation, and inhibiting viral replication and tumor cell growth [5]. TNF-α induces tumor cell apoptosis and modulates immune cell activity and inflammation. NCR3 plays a critical regulatory role in the immune defense and immune surveillance processes by modulating the secretion of these cytokines.
2.3 Impact on Immune Cell Interactions
NCR3's function extends beyond NK cell activation and cytotoxicity to include pivotal roles in interactions between immune cells. For example, when NCR3 binds to the Bcl-2 family anti-apoptotic gene 6 (BAG-6, also known as BAT3), it not only enhances NK cell killing of immature myeloid dendritic cells (DCs) but also induces NK cell secretion of TNF-α and IFN-γ. These cytokines further promote DC maturation. Such intercellular interactions strengthen the immune response network, enhancing the body's defense against pathogens and tumor cells.
3. NCR3 and Related Disease Research
3.1 NCR3 and Cancer
3.1.1 Tumor Immune Escape and NCR3
Cancer cells often evolve mechanisms to evade immune surveillance, including inhibiting NK cell function. Some tumor cells express ligands that bind to inhibitory receptors on NK cells, suppressing NK cell activity. However, NCR3 plays a unique role in tumor immunity. Studies have shown that B7-H6, a ligand expressed by tumor cells, binds to NCR3, with high expression rates observed in various solid tumors. For example, the positive rate of B7-H6 expression in colorectal cancer (CRC) is as high as 98%, in gastric cancer (GC) 77%, and in pancreatic ductal adenocarcinoma (PDAC) 63% [2]. Tumor cells expressing B7-H6 theoretically activate NCR3 on NK cells, enhancing tumor cell killing. However, in the tumor microenvironment, cancer cells may interfere with NCR3-mediated immune activation, leading to immune escape. For instance, immune suppressive factors in the tumor microenvironment may reduce NCR3 expression or function on NK cells, preventing effective recognition and elimination of tumor cells.
3.1.2 NCR3 as a Cancer Treatment Target
Given NCR3's critical role in tumor immunity, it has emerged as a potential target for cancer immunotherapy. Enhancing NCR3-mediated NK cell activity could overcome tumor immune escape and improve the immune system's ability to kill tumor cells. Current therapeutic strategies targeting NCR3 include using monoclonal antibodies to enhance NCR3-ligand binding or gene therapy to upregulate NCR3 expression on NK cells. Preclinical studies have shown that targeting NCR3 boosts NK cell anti-tumor activity, inhibits tumor growth, and extends survival in animal models [6] . NCR3-targeted cancer immunotherapy drugs are expected to provide new treatment options for cancer patients.
3.2 NCR3 and Infectious Diseases
3.2.1 Viral Infections and NCR3
NCR3 plays a vital role in the body's defense against viral infections. For instance, during vaccinia virus infections, target cells exhibit increased susceptibility to NK cell lysis, a process dependent on NCR3, NKp44, and NKp46. Human cytomegalovirus's major envelope protein pp65 can suppress NK cell cytotoxicity by interacting with NCR3, underscoring its importance in antiviral immunity [7]. In patients with chronic hepatitis C, the proportion of NK cells expressing NKp46 and NKp30 is significantly reduced compared to healthy individuals and those infected with hepatitis B, suggesting that changes in NCR3 expression may be linked to the progression of viral infections [8].
3.2.2 Role of NCR3 in Anti-Infection Immunity
When viral-infected cells express abnormal molecules or alter their molecular profiles, NCR3 can recognize these changes. Binding of NCR3 to ligands on infected cells activates NK cell cytotoxicity, prompting NK cells to lyse virus-infected cells and curtail viral replication and spread. Furthermore, NCR3 activation enhances NK cell secretion of cytokines like IFN-γ, which can regulate immune responses and increase the capacity of other immune cells to eliminate infected cells. Research into NCR3's role in anti-infection immunity could lead to new therapeutic strategies for viral infectious diseases.
3.3 NCR3 and Autoimmune Diseases
In autoimmune diseases like rheumatoid arthritis and multiple sclerosis, the immune system mistakenly attacks healthy tissues, leading to inflammation and tissue damage. NK cells play a crucial role in the pathogenesis of these diseases, and NCR3, as an activating receptor on NK cells, may influence disease progression. In theory, inhibiting NCR3 could reduce NK cell activity, alleviating immune attacks on healthy tissues, easing inflammation, and preventing disease progression. Studies have suggested that modulating NCR3 activity can regulate NK cell function in autoimmune diseases, offering new therapeutic insights [9]. However, further research is required to fully understand NCR3's role in autoimmune diseases and determine its viability as a therapeutic target.
4. Drug Research Progress
Currently, drug development targeting NCR3 focuses on two major frontiers: cancer immunotherapy and the regulation of infectious diseases. Some small molecules and biologics have entered clinical validation stages. Agonistic antibodies targeting NCR3 (such as NCR3-Agonist-01) combined with adoptive T-cell therapy have demonstrated significant enhancement of NK and T cell cytotoxicity against solid tumors, particularly showing durable response potential in refractory cancers like melanoma and non-small cell lung cancer. On the other hand, antagonistic NCR3 antibodies (such as NCR3-Antag-02) have proven effective in sepsis and chronic viral infection models by inhibiting the excessive activation of immune cells and the release of pro-inflammatory cytokines, thereby reducing the risk of tissue damage. Current research is focused on optimizing the engineering of the antibody Fc region to balance efficacy and safety, as well as exploring the synergistic mechanisms between NCR3 agonists and PD-1/L1 inhibitors or CAR-T cell therapies. Additionally, bispecific antibodies and cytokine fusion proteins based on the NCR3 signaling pathway are in early stages of development, offering promising new treatment strategies for immune escape-related tumors and immune storm-associated diseases.
|
Drug
|
Mechanism of ActionDrug Typ
|
Indications
|
Indications Developers
|
Developer Development Stages
|
|
NCR-300
|
Natural Killer (NK) Cell Therapy
|
Acute Myeloid Leukemia (AML) | Relapsed Myelodysplastic Syndromes (MDS) | Refractory Myelodysplastic Syndromes (MDS) | Myelodysplastic Syndromes (MDS) | Hematopoietic Stem Cell Transplantation (HSCT)
|
Nuwacell company
|
Phase I/II
|
|
BI-765049
|
Bispecific T-cell Engager (BiTE)
|
Colorectal Cancer|PDAC|Rectal Cancer| Gastric Cancer|Head and Neck Cancer|Liver Cancer|NSCLC|Pancreatic Cancer|Tumors|Metastatic Colorectal Cancer
|
Boehringer Ingelheim GmbH | Boehringer Ingelheim International GmbH
|
Phase I
|
|
IAN-0982
|
Bispecific NK-cell Engager (BiTE)
|
Tumors
|
SUNHO biopharmaceutical(china)co.lto
|
Preclinical
|
|
CTX-8573
|
Trispecific NK Cell Engager
|
Multiple Myeloma
|
Compass Therapeutics, Inc.
|
Preclinical
|
|
CTX-4419
|
Bispecific Antibody
|
Multiple Myeloma
|
Compass Therapeutics LLC | Compass Therapeutics, Cambridge, MA, USA
|
Preclinical
|
5. NCR3-Related Products Recommendation
As a critical activating receptor on the surface of NK cells, NCR3's unique structure plays a key role in immune regulation. A deeper understanding of NCR3's structure, mechanisms of action, and its association with related diseases provides a solid theoretical foundation for developing novel therapeutic strategies for a variety of diseases, including cancer, infectious diseases, and autoimmune disorders. CUSABIO offers high-quality NCR3-related proteins and antibodies to support researchers in their studies.
● NCR3 Antibodies
NCR3 Antibody; CSB-PA015551LA01HU
IHC
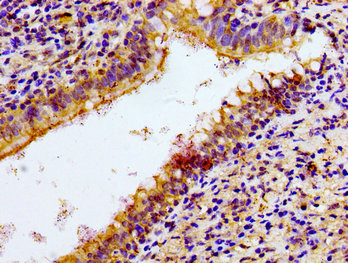
Immunohistochemistry of paraffin-embedded human lung cancer using CSB-PA015551LA01HU at dilution of 1:100
IF
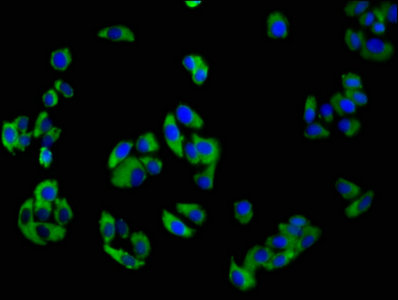
Immunofluorescence staining of HepG2 cells with CSB-PA015551LA01HU at 1:100, counter-stained with DAPI. The cells were fixed in 4% formaldehyde, permeabilized using 0.2% Triton X-100 and blocked in 10% normal Goat Serum. The cells were then incubated with the antibody overnight at 4°C. The secondary antibody was Alexa Fluor 488-congugated AffiniPure Goat Anti-Rabbit IgG(H+L).
References
[1] Moretta A, Bottino C, Vitale M, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis[J]. Annual review of immunology, 2001, 19(1): 197-223.
[2]Brandt C S, Baratin M, Yi E C, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans[J]. Journal of Experimental Medicine, 2009, 206(7): 1495-1503
[3] von Strandmann E P, Simhadri V R, von Tresckow B, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells[J]. Immunity, 2007, 27(6): 965-974.
[4] Sivori S, Vitale M, Morelli L, et al. p46, a novel natural killer cell–specific surface molecule that mediates cell activation[J]. The Journal of experimental medicine, 1997, 186(7): 1129-1136.
[5] Koch J, Steinle A, Watzl C, et al. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection[J]. Trends in immunology, 2013, 34(4): 182-191.
[6] Fend L, Rusakiewicz S, Adam J, et al. Prognostic impact of the expression of NCR1 and NCR3 NK cell receptors and PD-L1 on advanced non-small cell lung cancer[J]. Oncoimmunology, 2017, 6(1): e1163456.
[7] Arnon T I, Achdout H, Levi O, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus[J]. Nature immunology, 2005, 6(5): 515-523.
[8] Li H J, Yang N, Mu X, et al. Reduction of natural killer cells is associated with poor outcomes in patients with hepatitis B virus-related acute-on-chronic liver failure[J]. Hepatology International, 2022, 16(6): 1398-1411.
[9] Gao B. Identification of feature autophagy-related genes and DNA methylation profiles in systemic lupus erythematosus patients[J]. Medical science monitor: international medical journal of experimental and clinical research, 2021, 27: e933425-1.
CUSABIO team. Immune Battlefield's "Scout": Decoding the NCR3 Target. https://www.cusabio.com/c-21236.html

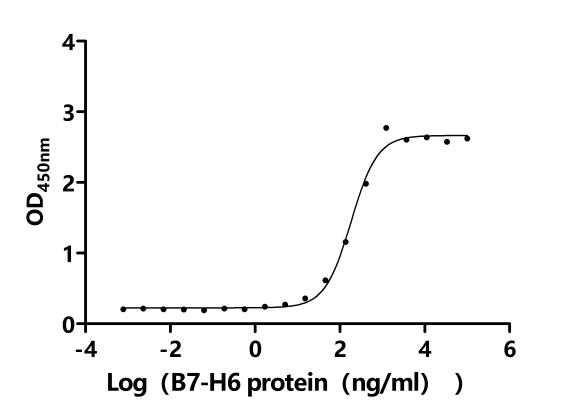





Comments
Leave a Comment