1. What is Interleukin-2 (IL2)?
Interleukin-2 (IL2) is a key immunoregulatory cytokine initially discovered for its ability to promote T cell proliferation. It plays a “double-edged sword” role in the immune system balance: it can both activate effector T cells (Teff) to mediate antitumor and anti-infection immunity and maintain the function of regulatory T cells (Treg) to suppress excessive immune responses [1].
Early studies showed that high-dose IL2 (HD-IL2) can activate cytotoxic T cells and natural killer (NK) cells to exert antitumor effects and was the first immunotherapy approved for metastatic renal cell carcinoma (mRCC) and melanoma [2,3]. Low-dose IL2, on the other hand, is used for the treatment of autoimmune diseases and transplant rejection due to its preferential expansion of Treg cells (which express the high-affinity IL2 receptor CD25) [4,5]. In recent years, the combination of IL2 with other immunomodulators and the development of new formulations have become research hotspots [2,6].
2. Research Mechanism
The biological functions of IL2 depend on its binding to the IL2 receptor (IL2R), with effects that are dose-dependent and cell type-specific, as detailed below:
2.1 IL2 Receptor Subtypes and Functional Differences
The IL2R is composed of CD25 (α-chain), CD122 (β-chain), and CD132 (γ-chain), forming three types of receptors through different combinations:
2.1.1 High-affinity receptor (CD25+CD122+CD132)
Expressed only on Treg cells, this receptor has the highest affinity for IL2 (Kd≈10⁻¹¹ M). Low-dose IL2 (e.g., 0.3-1×10⁶ IU/m²) can continuously activate Treg cells through this receptor, promoting their proliferation, survival, and functional maintenance (e.g., stable expression of FOXP3) [4,5]. For example, in patients with chronic graft-versus-host disease (GVHD), after 4 weeks of low-dose IL2 treatment, the number of Treg cells increased more than 8-fold compared to baseline, and the proportion of FOXP3+ cells significantly increased [4].
2.1.2 Medium-affinity receptor (CD122+CD132)
Expressed on Teff, NK cells, and memory T cells, this receptor has moderate affinity (Kd≈10⁻⁹ M). High-dose IL2 (e.g., 600,000-720,000 IU/kg) preferentially activates this receptor, enhancing the expression of cytotoxic molecules (such as perforin and granzymes) and thereby increasing the antitumor/anti-infection activity of Teff and NK cells [3,7].
2.1.3 Low-affinity receptor (CD122+CD132, without CD25)
With the lowest affinity (Kd≈10⁻⁷ M), this receptor mainly functions in the later stages of immune activation, participating in the formation of immunological memory [1].
2.2 Dose-Dependent Immune Modulatory Effects
2.2.1 Immune activation by high-dose IL2 (HD-IL2)
- Stimulates the proliferation and differentiation of Teff cells: Enhancing their recognition and killing of tumor cells. In melanoma research, the combination of HD-IL2 and gp100 vaccine increased the number of antigen-specific CD8+ T cells, raising the objective response rate (ORR) from 6% to 16% [3].
- Activates NK cells: HD-IL2 promotes the secretion of IFN-γ by NK cells, enhancing their antibody-dependent cellular cytotoxicity (ADCC) against tumor cells. For example, in a neuroblastoma model, the combination of IL2 and anti-GD2 antibody significantly increased the cytotoxic activity of NK cells against tumor cells [8].
2.2.2 Immune suppression by low-dose IL2:
- Selectively expands Treg cells: By maintaining the expression of FOXP3 and inhibitory functions of Treg cells, it reduces excessive immune responses. In HCV-induced vasculitis, low-dose IL2 increased the proportion of Treg cells among CD4+ T cells from 5% to 15% at baseline, while inhibiting the secretion of pro-inflammatory cytokines (such as IL-17 and TNF-α) [9,10].
- Regulates immune balance: By increasing the Treg:Tcon (conventional T cell) ratio (from 0.07 to 0.39), it inhibits the activation of autoreactive T cells. In patients with chronic GVHD, an elevated ratio was significantly associated with the improvement of symptoms such as skin sclerosis and liver dysfunction [4].
2.3 Synergistic Mechanisms with Other Molecules
- Combination with vaccines: The gp100 peptide vaccine activates antigen-specific T cells, while IL2 provides co-stimulatory signals to promote their proliferation. The combination extended the median overall survival of melanoma patients from 11.1 months to 17.8 months [3].
- Combination with HDAC inhibitors: Entinostat inhibits the immunosuppressive functions of Treg cells (e.g., reducing IL-10 secretion). When combined with HD-IL2, the ORR in mRCC patients increased from 20-25% to 37%, and the median progression-free survival (PFS) extended from 4.2 months to 13.6 months [2].
- Combination with immune checkpoint inhibitors: Low-dose ipilimumab (anti-CTLA-4) can relieve the immunosuppression of T cells and, in combination with HD-IL2, synergistically activate Teff cells. This has achieved an objective response rate (ORR) of 18.2% in immune-resistant melanoma, with some patients experiencing durable responses lasting over 37 months [7].
3. Signal Pathways
IL2 activates specific signaling pathways through binding to different types of receptors, with effects significantly varying depending on cell type and IL2 dose:
3.1 JAK-STAT Pathway (Primarily Regulates Treg Function)
When IL2 binds to the high-affinity receptor (CD25+CD122+CD132), it recruits and activates JAK1 and JAK3 kinases, leading to the phosphorylation of STAT5 and the formation of dimers that enter the nucleus to regulate the expression of genes such as FOXP3 and CD25. This is the core pathway for maintaining the survival, proliferation, and function of Treg cells [8,15]. Low-dose IL2 preferentially activates this pathway. In patients with chronic GVHD, after 4 weeks of treatment, the expression of FOXP3 in Treg cells increased 2.3-fold compared to baseline and was maintained with continued treatment [4].
3.2 PI3K-Akt Pathway (Primarily Regulates Cell Proliferation and Survival)
This pathway is mainly mediated by the medium-affinity receptor (CD122+CD132). After IL2 binds, it activates PI3K through Src family kinases, generating PIP3 and recruiting Akt for phosphorylation. This process inhibits pro-apoptotic molecules (such as Bad) and activates proliferation-related genes (such as Cyclin D1), ultimately promoting the survival and proliferation of Teff and NK cells [1]. In melanoma treatment, high-dose IL2 increased the level of phosphorylated Akt in Teff cells by 1.8-fold and the proliferation index (Ki67+) by 40%, directly enhancing the antitumor immune response [3].
3.3 MAPK Pathway (Auxiliary Regulation of Immune Cell Activation)
IL2 activates this pathway through the Ras-Raf-MEK-ERK cascade, especially significantly in Teff cells treated with high-dose IL2. After ERK is phosphorylated and enters the nucleus, it promotes the transcription of cytokines such as IL-2 and IFN-γ, enhancing the cytotoxic function of effector T cells [1]. For example, in the treatment combining gp100 vaccine and IL2, the secretion of IFN-γ by Teff cells increased 2.1-fold compared to the IL2 monotherapy group, directly correlating with enhanced tumor-killing activity [3].
3.4 Cross-Regulation of Pathways
The balance between the JAK-STAT and PI3K-Akt pathways determines the direction of the immune response: low-dose IL2 preferentially activates the JAK-STAT pathway (Treg dominant), while high-dose IL2 simultaneously activates the PI3K-Akt pathway (Teff dominant) [1]. Additionally, STAT5 activated by IL2 can synergize with the NF-κB pathway in NK cells to upregulate the expression of cytotoxic molecules such as perforin, further enhancing immune effects [8].
4. Related Diseases
Abnormal regulation of IL2 can lead to immune imbalance and disordered signaling pathways, which are closely related to the occurrence and development of various diseases:
4.1 Malignant Tumors
4.1.1 Renal Cell Carcinoma (RCC)
Weakening of IL2 signaling in the tumor microenvironment is an important mechanism for immune evasion in RCC. Tumor cells can secrete inhibitory factors such as IL-10, downregulating the expression of CD122 on T cells, thereby reducing the sensitivity of Teff cells to IL2 and weakening the antitumor immune response [2,11]. The ORR of HD-IL2 monotherapy for metastatic clear cell RCC is 20-25%, with a median PFS of 4.2 months. However, patients who achieve complete remission can have a survival period of over 5 years. When combined with entinostat, the ORR increases to 37%, and the median PFS extends to 13.6 months. This is because entinostat can reverse the immunosuppressive function of Treg cells and enhance the activation of Teff cells by IL2 [2,11].
4.1.2 Melanoma
Some melanoma cells inhibit the expression of IL2 pathway-related genes (such as JAK3 and STAT5) through mutations or epigenetic modifications, blocking the proliferation of Teff cells. Meanwhile, tumor-infiltrating Treg cells highly express CD25, competitively binding IL2 and further weakening the immune response [3,7]. The ORR of HD-IL2 combined with gp100 peptide vaccine in HLA-A0201-positive patients is 16%, higher than the 6% achieved with HD-IL2 monotherapy. This is because the antigen-specific T cells activated by the vaccine are more responsive to the proliferative signals of IL2. For immune-resistant patients, the ORR of HD-IL2 combined with low-dose ipilimumab and nivolumab is 18.2%. This is because checkpoint inhibitors can release the “brakes” on T cells, and in combination with IL2, synergistically restore antitumor activity [3,7].
4.1.3 Neuroblastoma
Neuroblastoma cells highly express the GD2 antigen on their surface, but the IL2 concentration in their microenvironment is insufficient, limiting the ADCC effect of NK cells [8]. The combination of dinutuximab, IL2, and GM-CSF can enhance the ADCC effect of NK cells. However, the SIOPEN trial showed that compared to dinutuximab alone, the addition of IL2 did not significantly improve the 3-year event-free survival rate (56% vs. 60%), and the incidence of grade 3-4 capillary leak syndrome was higher (11-13% vs. 3-6%). This may be due to the excessive activation of monocytes by IL2, leading to the release of inflammatory cytokines [8].
4.2 Autoimmune and Transplant-Related Diseases
4.2.1 Chronic Graft-Versus-Host Disease (GVHD)
After allogeneic hematopoietic stem cell transplantation, donor-derived Teff cells attack host tissues due to IL2 signal hyperactivity. Meanwhile, Treg cells have defects in number or function (such as unstable FOXP3 expression) and cannot effectively suppress excessive immunity [4]. For patients refractory to glucocorticoids, daily subcutaneous injection of IL2 (1×10⁶ IU/m²) for 8 weeks resulted in partial remission in 52% of patients. This is because IL2 preferentially expands Treg cells, increasing the Treg:Tcon ratio from 0.07 to 0.39 and restoring immune balance [4].
4.2.2 HCV-Induced Vasculitis
HCV infection induces B cells to produce cryoglobulin, activating the complement system and neutrophils. Meanwhile, Treg cells are impaired in function (reduced activity of the IL2-JAK-STAT5 pathway), leading to the overactivation of autoreactive T cells [9,10]. After treatment with IL2 in 10 patients, 8 showed symptom improvement. This is because IL2 enhanced the expression of FOXP3 and inhibitory functions of Treg cells, reducing the expression of inflammatory genes (TNF-α, IL-6) [9,10].
4.2.3 Multiple Sclerosis (MS)
In MS patients, T cells infiltrating the central nervous system have abnormally elevated sensitivity to IL2 and proliferate excessively through the PI3K-Akt pathway. Meanwhile, NK cells have impaired function due to insufficient IL2 supply and cannot clear autoreactive T cells [12]. The anti-CD25 antibody (daclizumab) can block the binding of IL2 to Treg cells, allowing free IL2 to preferentially activate NK cells and reduce T cell infiltration. s have shown that it reduces the annual relapse rate from 0.46 to 0.21 [12].
4.3 Infectious Diseases
4.3.1 HIV Infection
HIV targets and destroys CD4+ T cells, leading to reduced IL2 secretion. Meanwhile, viral proteins (such as Nef) can interfere with the assembly of IL2R, weakening the response of remaining CD4+ T cells to IL2 and impeding immune reconstitution [13]. IL2 can promote the proliferation of CD4+ T cells. However, the INSIGHT-ESPRIT trial showed that IL2 combined with antiretroviral therapy did not reduce the risk of opportunistic infections or death. Only in the subgroup with baseline CD4+ T cell counts < 200/μL did IL2 treatment reduce the primary endpoint events by 22%, as these low-level CD4+ T cells are more dependent on IL2 for recovery [13].
5. Recent Research Advances in IL-2-Related Drugs
Recently, significant progress has been made in the development of IL-2-related drugs. Rezpegaldesleukin, developed by Nektar Therapeutics, has successfully completed a Phase 2b for the treatment of moderate to severe atopic dermatitis, significantly reducing the Eczema Area and Severity Index (EASI) with good safety. Teva and Fosun Pharma have partnered to jointly develop TEV-56278, an immunotherapy that fuses an anti-PD-1 antibody with IL-2, aiming to enhance antitumor activity while reducing toxicity. Asher Bio's IL-2 candidate drug AB248 is also undergoing Phase 1a/1b s for the treatment of various advanced solid tumors and is planned to be combined with bispecific T cell engagers for small cell lung cancer. Additionally, several IL-2 drugs are in preclinical or clinical research stages, some of which are listed below:
| drugs |
Mechanism of action |
Type of medication |
Indications under investigation (disease name) |
Institutions under research |
Highest R&D stage |
| NuLeusin |
IL-2 stimulators |
Interleukin |
Myeloproliferative disorders | Metastatic renal cell carcinoma |
3SBio, Inc |
Application for listing |
| Interleukin-2 inhalation(Immunservice GmbH) |
IL-2 stimulators |
Interleukin |
Renal cell carcinoma |
Immunservice GmbH |
Phase 3 |
| Saltikva |
IL-2 substitutes |
Live bacteria preparations |
Pancreatic cancer | Metastatic pancreatic cancer |
Salspera LLC |
Phase 2 |
| COYA-301 |
IL-2 modulators |
Interleukin |
Alzheimer's Disease | Amyotrophic lateral sclerosis | Frontotemporal dementia |
Coya Therapeutics, Inc. |
Phase 2 |
| BNZ-1 |
IL-15 inhibitors | IL-2 inhibitor | IL-9 inhibitors |
Synthetic peptides |
Alopecia areata | Total baldness | Generalized alopecia | Cutaneous T-cell lymphoma | Lymphocytic leukemia |
Equillium, Inc. | National Institutes of Health | Bioniz Therapeutics, Inc. |
Phase 2 |
| PT101 |
IL-2 modulators |
Fc fusion protein |
Systemic lupus erythematosus | Non-segmental vitiligo | Moderate atopic dermatitis | Severe atopic dermatitis | Ulcerative colitis |
Merck Sharp & Dohme LLC | Merck R&D (China) Co., Ltd. | Merck Sharp & Dohme Corp. |
Phase 2 |
| Oncoquest-L vaccine(Xeme Biopharma) |
IL-2 Substitute | Immunomodulators |
Therapeutic vaccines |
Recurrent grade 3a follicular lymphoma |
XEME Biopharma, Inc. |
Phase 2 |
| KB-707 |
IL-12 modulator | IL-2 modulators |
Cytokines |
Advanced malignant solid tumors | Non-small cell lung cancer | Cutaneous melanoma |
Krystal Biotech, Inc. |
Phase 1/2 |
| IOV-3001 |
IL-2 substitutes |
Recombinant proteins |
Locally advanced melanoma | Metastatic melanoma | Unresectable melanoma | Uveal melanoma |
Iovance Biotherapeutics, Inc. |
Phase 1/2 |
| Polyethylene glycolated recombinant human interleukin-2 (Mabwell Bioscience) |
IL-2 inhibitors |
Interleukin |
Advanced malignant solid tumors |
Mabwell (Shanghai) Biotechnology Co., Ltd |
Phase 1/2 |
| EGL-001 |
CTLA4 inhibitor | IL-2 inhibitors |
Cytokines | Antibody fusion proteins |
Locally advanced malignant solid tumors |
Egle Therapeutics SAS |
Phase 1/2 |
| Igrelimogene litadenorepvec |
IL-2 inhibitor | TNF-α inhibitors |
Oncolytic virus |
Platinum-resistant fallopian tube cancer | platinum-resistant ovarian cancer, etc |
TILT Biotherapeutics Oy | Biotheus Biopharmaceutical (Nantong) Co., Ltd |
Phase 1/2 |
| IPH-6501 |
CD16a modulator | CD20 modulator | IL-2 modulator | NKp46 inhibitors |
NK cell sagger| Four-specific antibodies |
CD20-positive B-cell non-Hodgkin lymphoma | Diffuse large B-cell lymphoma, etc |
Innate Pharma SA |
Phase 1/2 |
| SAR-444336 |
IL-2 modulators |
Interleukin |
inflammation |
Sanofi |
Phase 1 |
| ASKG322 |
IL-2 inhibitors |
Fusion proteins |
tumor |
Jiangsu Aosaikang Pharmaceutical Co., Ltd. | AskGene Pharma, Inc. |
Phase 1 |
| AGX-148 |
IL-12 Substitute | IL-2 Substitute | Immunocytotoxicity | T lymphocyte substitutes |
Tumors infiltrate lymphocytes |
Advanced malignant solid tumors | Colorectal cancer | Tumors of the female reproductive organs | Lung Cancer | Melanoma | Squamous cell carcinoma of the head and neck |
AgonOx LLC | SQZ Biotechnologies Co. |
Phase 1 |
| CLN-617 |
IL-12 Substitute | IL-2 substitutes |
Fusion proteins |
Advanced malignant solid tumors | Locally advanced melanoma | Squamous cell carcinoma of the head and neck | Unresectable melanoma |
Cullinan Oncology LLC |
Phase 1 |
| VIS171 |
IL-2 Substitute | IL-2R Modulator | Modulation of T-lymphocyte stimulators |
Interleukin |
Alopecia areata | Systemic lupus erythematosus | Autoimmune diseases |
Otsuka Pharmaceutical Development & Commercialization, Inc. | Visterra, Inc.
|
Phase 1 |
| BNT-153 |
IL-2 Stimulator | Immunostimulants |
mRNA |
Advanced malignant solid tumors |
BioNTech SE |
Phase 1 |
| Interleukin-2 gene therapy(St. Jude Children's Research Hospital, Inc.) |
IL-2 stimulators |
Gene therapy |
Neuroblastoma |
St. Jude Children's Research Hospital, Inc. |
Phase 1 |
| Cord Blood-derived IL-10 CD19-CAR NK(The Second Affiliated Hospital Zhejiang University School of Medicine) |
CD19 modulator | IL-2 modulators |
CAR-NK |
Mediastinal large B-cell lymphoma |
The Second Affiliated Hospital of Zhejiang University School of Medicine (Zhejiang Provincial Second Hospital) |
Phase 1 |
| VET2-L2 |
CCR2 modulators | IL-2 modulator | Leptin modulator |
Oncolytic virus |
Acral freckles malignant melanoma | BRAF mutation-positive melanoma | Gastroesophageal junction carcinoma | Locally advanced malignant solid tumors, etc |
Astellas Pharma Global Development, Inc. | Kalivir Immunotherapeutics, Inc. | Astellas Pharma, Inc. |
Phase 1 |
| IMD-101 |
IL-2 inhibitors |
Biologics |
Bladder Cancer | Kidney tumors | Melanoma | Mesothelioma | Non-small cell lung cancer | Solid tumors | Advanced malignant solid tumors |
Shanghai Affinity Biomedical Technology Co., Ltd. | Catalysis Therapeutics Pty Ltd |
Shanghai Yingmaide Medical Technology Co., Ltd |
Phase 1 |
| WTX-124 |
IL-2 substitutes |
Cytokines |
Advanced malignant solid tumors | Cutaneous melanoma | Metastatic solid tumors |
Werewolf Therapeutics, Inc. |
Phase 1 |
| TEV-56278 |
IL-2 modulator | PD-1 inhibitors |
Antibody fusion proteins |
Locally advanced malignant solid tumors | Metastatic solid tumors | tumor |
Teva Pharmaceutical Industries Ltd. | Teva Branded Pharmaceutical Products R&D, Inc. |
Phase 1 |
| RS-2102 |
IL-2 substitutes |
Protein drugs |
Atopic dermatitis |
Jiangsu Hengrui Pharmaceutical Co., Ltd |
Phase 1 |
| PM-1016 |
IL-2 inhibitor | Stimulator of cell death |
Gene therapy |
hepatocellular carcinoma |
TILT Biotherapeutics Oy |
Clinical application approval |
| NCB-003 |
IL-2 substitutes |
Recombinant proteins |
Advanced malignant solid tumors |
Zhejiang Xinma Biopharmaceutical Co., Ltd |
Clinical application approval |
| CUG-288 |
IL-2 Substitute | T lymphocyte stimulators |
Fusion proteins |
Solid tumors |
Cugene, Inc. |
Clinical applicatio |
*Data sourced from Patsnap
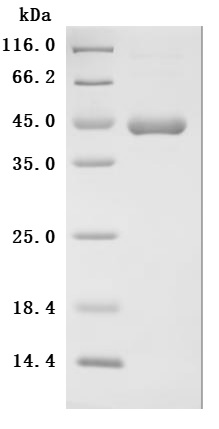
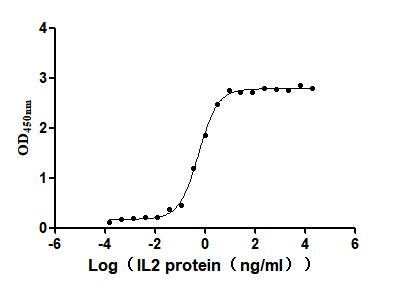
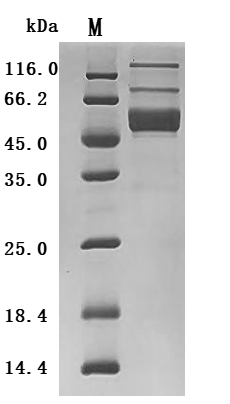
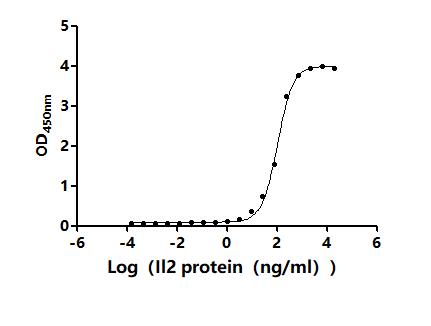
6. CUSABIO IL-2 Related Product Recommendations
References
[1] Bluestone JA. The Yin and Yang of Interleukin-2–Mediated Immunotherapy. N Engl J Med. 2011;365:2129-2131.
[2] Pili R, et al. A randomized, open-label, phase II study of high-dose interleukin 2 vs high-dose interleukin 2 plus entinostat in renal cell carcinoma. 2023.
[3] Schwartzentruber DJ, et al. gp100 Peptide Vaccine and Interleukin-2 in Patients with Advanced Melanoma. N Engl J Med. 2011;364:2119-2127.
[4] Koreth J, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055-2066.
[5] Zhao X, et al. Prophylactic use of low-dose interleukin-2 early post-transplantation: a randomised study. 2015.
[6] Amaria RN, et al. OBX-115, an interleukin 2-sparing engineered tumor-infiltrating lymphocyte cell therapy, in patients with immune checkpoint inhibitor-resistant melanoma. 2025.
[7] Tarhini AA, et al. High dose bolus interleukin-2 and concurrent low dose ipilimumab followed sequentially by nivolumab in patients with advanced melanoma. 2025.
[8] Matthay KK. Interleukin 2 plus anti-GD2 immunotherapy: helpful or harmful? Lancet Oncol. 2018;19:1549-1551.
[9] Oo YH, et al. Low-Dose Interleukin-2 and HCV-Induced Vasculitis. N Engl J Med. 2012;366:1353-1354.
[10] Saadoun D, et al. Regulatory T-Cell Responses to Low-Dose Interleukin-2 in HCV-Induced Vasculitis. N Engl J Med. 2011;365:2067-2077.
[11] Chow S, et al. HIGH-DOSE INTERLEUKIN-2 ARMED WITH PATHOLOGY-BASED SELECTION CRITERIA: A REAL OPTION IN TREATMENT OF METASTATIC RENAL CELL CARCINOMA. Ann Oncol. 2014;25(suppl 4):iv294.
[12] Saidha S, et al. Anti-interleukin-2 receptor alpha for multiple sclerosis? Lancet. 2013;381:2141-2142.
[13] Sellier P, et al. Interleukin-2 Therapy in Patients with HIV Infection. N Engl J Med. 2010;362:270-271.
CUSABIO team. Interleukin-2 (IL2): The "Double-Edged Sword" of the Immune System. https://www.cusabio.com/c-21248.html

.png)


Comments
Leave a Comment