[1] Barashdi, M., Ali, A., McMullin, M., & Mills, K. (2021). Protein tyrosine phosphatase receptor type C (PTPRC or CD45). Journal of Clinical Pathology, 74, 548 - 552.
[2] Rheinländer, A., Schraven, B., & Bommhardt, U. (2018). CD45 in human physiology and clinical medicine.. Immunology letters, 196, 22-32.
[3] Borowska, M., Liu, L., Caveney, N., et al. (2024). Orientation dependent CD45 inhibition with viral and engineered ligands. Science immunology, 9, eadp0707 - eadp0707.
[4] Dunlock, V., Arp, A., Singh, S.,et al. (2022). Tetraspanin CD53 controls T cell immunity through regulation of CD45RO stability, mobility, and function.. Cell reports, 39 13, 111006.
[5] Gil-Cantero, S., Puck, A., Künig, S., et al. (2025). The Soluble Cytoplasmic Tail of CD45 (ct-CD45) Regulates Dendritic Cell Activation and Function via TLR4 Signaling. International Journal of Molecular Sciences, 26.
[6] Lui, Y., Fernandes, J., Vuong, M., Sharma, S., et al. (2025). The Structural Biology of T‐Cell Antigen Detection at Close Contacts. Immunological Reviews, 331.
[7] Chang, V., Fernandes, R., Ganzinger, K., et al. (2016). Initiation of T cell signaling by CD45 segregation at ‘close-contacts’. Nature immunology, 17, 574 - 582.
[8] Kumar, V., Cheng, P., Condamine, T., et al. (2016). CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation.. Immunity, 44 2, 303-15.
[9] Park, S., Kim, J., Jang, G., et al. (2021). Aberrant activation of the CD45-Wnt signaling axis promotes stemness and therapy resistance in colorectal cancer cells. Theranostics, 11, 8755 - 8770.
[10] Ahmed, M., Limmer, A., & Hartmann, M. (2023). CD45RA and CD45RO Are Regulated in a Cell-Type Specific Manner in Inflammation and Sepsis. Cells, 12.
[11] Espinoza-Gutarra, M., Agarwal, P., Ferrer, L., et al. (2020). Relationship between CD45 Expression and Outcomes in B Lymphoblastic Leukemia/Lymphoma. Blood, 136, 24-24.
[12] Peng, Q., Zhu, B., Lu, Y., et al.(2023). Abstract 16746: Novel Role of Endothelial CD45 in Regulating Endothelial-to-Mesenchymal Transition (EndMT) in Atherosclerosis. Circulation.
[13] Alon, D., Paitan, Y., Robinson, E.,et al.(2021). Downregulation of CD45 Signaling in COVID-19 Patients Is Reversed by C24D, a Novel CD45 Targeting Peptide. Frontiers in Medicine, 8.

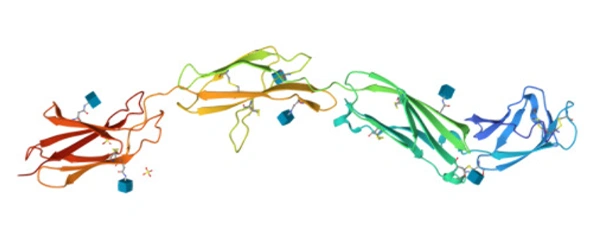

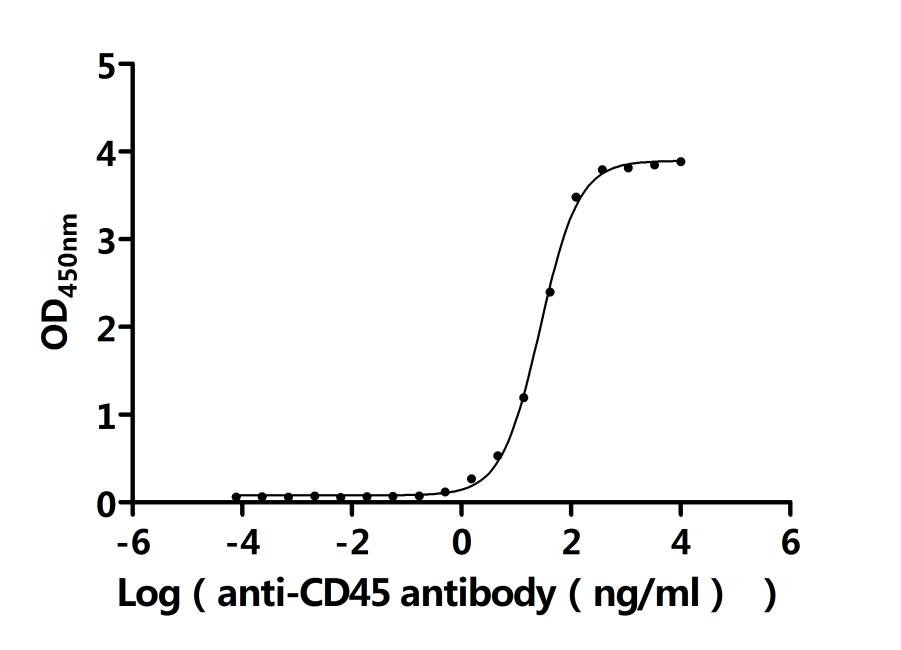
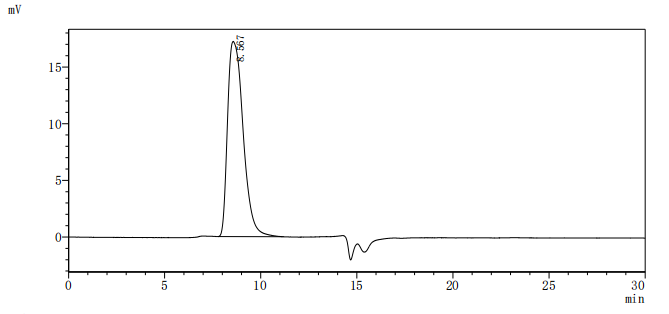
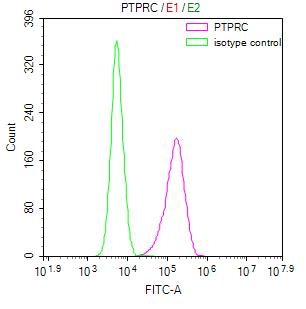



Comments
Leave a Comment