On September 29, Genmab announced it had entered into an acquisition agreement with Merus to purchase all outstanding shares of the latter for $97 per share, totaling approximately $8 billion. The LGR5/EGFR bispecific antibody Petosemtamab was the primary target of this acquisition by Genmab. Currently in Phase 3 clinical development, Petosemtamab demonstrates significant anti-tumor activity by simultaneously targeting EGFR and LGR5, enabling more precise identification and attack of tumor cells.
This acquisition has brought LGR5 back into the spotlight. From intestinal regeneration to colorectal cancer treatment, LGR5 is reshaping our understanding of "stem cell fate." This article will systematically explore how this molecule connects stem cells and cancer, revealing the underlying signaling pathways and new advances in drug development.
1. Molecular Structure and Functional Characteristics of LGR5
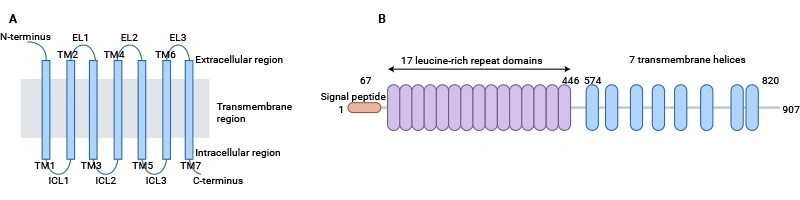
LGR5 (Leucine-rich repeat-containing G protein-coupled receptor 5) belongs to the leucine-rich repeat-containing G protein-coupled receptor (GPCR) family. Its typical structure includes a seven-transmembrane domain and an extracellular N-terminal ligand-binding region rich in leucine repeats (LRR) [1]. This structure enables LGR5 to perform dual functions: ligand recognition and signal transduction. The LRR domain, typically composed of 18-25 repeat units, forms a stable structure via β-sheet layers and can specifically bind R-spondin (RSPO) family ligands; the transmembrane domain activates downstream G protein signal transduction through conformational changes [2].
Fig 1. Schematic presentation of the general structure of GPCRs and LGR5 [1]
Studies show that the third intracellular loop of LGR5 contains multiple phosphorylation sites, enabling recruitment of β-arrestin to mediate signal internalization, playing a dynamic regulatory role in pathways such as Wnt/β-catenin [3]. In signal transduction, LGR5 enhances Wnt signaling intensity through a dual mechanism: on one hand, the LGR5-RSPO complex binds to the Wnt co-receptors LRP5/6, inhibiting the internalization and degradation of Wnt receptors by the E3 ubiquitin ligases RNF43/ZNRF3, thereby prolonging signal activation time; on the other hand, coupling of the LGR5 Gαs subunit can activate the cAMP-PKA pathway, enhancing the transcriptional activity of β-catenin via phosphorylation at Ser675 [4].
Single-cell sequencing results indicate that Wnt target genes AXIN2 and LEF1 are expressed significantly higher in LGR5-positive stem cells than in ordinary cells, suggesting a signal amplification function [1]. Furthermore, the PDZ-binding motif at the intracellular C-terminus of LGR5 can form complexes with scaffold proteins like DLG1, further regulating cell polarity and migration [3].
At the tissue level, LGR5 exhibits a distinct specific expression pattern. In intestinal crypt base columnar cells, LGR5 regulates the self-renewal and differentiation of stem cells by maintaining the Wnt signaling gradient [2]; In the hair follicle bulge, LGR5 synergizes with BMP signaling to control the hair follicle regeneration cycle[4]. Additionally, some splice variants of LGR5 (e.g., soluble forms) can competitively bind ligands, producing a negative regulatory effect; these isoforms are abnormally highly expressed in colorectal cancer and are closely related to the maintenance of tumor stemness. Cryo-EM structural analysis reveals that key residues like Arg72 and Glu110 at the LGR5-RSPO2 binding interface provide a structural basis for designing targeted inhibitors [3].
2. The Role of LGR5 in Tissue Development and Homeostasis Maintenance
The structural characteristics of LGR5 determine its important functions in tissue development and homeostasis maintenance. Its extracellular domain contains 17 leucine-rich repeats, which can bind RSPO family ligands and activate downstream signaling pathways. Studies have found that LGR5 is highly expressed in intestinal crypt base stem cells, promoting stem cell self-renewal and balanced differentiation by maintaining Wnt/β-catenin pathway activity, playing a key role in the continuous regeneration of the intestinal epithelium [5]. In liver tissue, LGR5-positive cells rapidly proliferate after injury, promoting biliary epithelial repair and regeneration. In the skin system, LGR5 is cyclically expressed in hair follicle bulge stem cells, maintaining the hair follicle regeneration cycle.
During embryonic development, LGR5 participates in the directed differentiation of mesenchymal stem cells; knockout models show its absence leads to abnormal structure of the long bone growth plate. In adult tissue repair, LGR5-positive cells demonstrate migratory and multi-directional differentiation potential. For example, in myocardial infarction models, epicardial LGR5+ cells can transdifferentiate into cardiomyocytes and vascular endothelial cells, promoting tissue reconstruction. Furthermore, LGR5 expression levels are closely related to stemness; its downregulation leads to depletion of the stem cell pool and accelerates tissue aging.
In the interaction between gut microbiota and the host, LGR5 maintains intestinal barrier function by regulating bile acid metabolism; abnormal expression is associated with increased mucosal permeability and enhanced inflammatory responses [2]. Research on adipose tissue in patients with obstructive sleep apnea also indicates that changes in LGR5 expression work in concert with other factors to regulate lipid metabolism disorders, suggesting its important role in metabolic homeostasis [2].
3. LGR5-Mediated Wnt/β-catenin Signaling Pathway and Cross-Regulatory Mechanisms
3.1 Mechanism of Wnt/β-catenin Signaling Pathway Activation
LGR5 enhances the stability of Wnt ligand binding to Frizzled receptors by binding R-spondin protein family members and forming an LGR5–RSPO–Wnt receptor complex, thereby inhibiting RNF43/ZNRF3-mediated degradation of Wnt receptors and prolonging the activation of Wnt/β-catenin signaling [6]. This regulation is a key mechanism in cell fate determination and stemness maintenance. Structural studies show that the 17 leucine-rich repeat units of LGR5 form a horseshoe-shaped binding domain, providing a high-affinity binding site for RSPO [5].
LGR5-mediated Wnt signal regulation exhibits dose-dependent characteristics. The presence of R-spondin can increase the signal intensity mediated by LGR5 more than tenfold, while loss of LGR5 completely prevents intestinal organoid formation [7]. Its third intracellular loop (ICL3) can regulate the membrane stability of the Wnt receptor Frizzled and participate in the recruitment of E3 ubiquitin ligases [8].
After nuclear β-catenin binds to the TCF/LEF complex, it can upregulate the transcription of target genes such as MYC, CCND1, and AXIN2, regulating cell proliferation and differentiation. Furthermore, the β-catenin/TCF complex can also bind TCF-binding elements on the LGR5 promoter, forming a positive feedback loop that continuously amplifies pathway activity [9].
Epigenetic regulation is also involved in LGR5-related signal activation. ChIP sequencing results show enrichment of H3K27ac modification in LGR5-highly expressing cells and decreased activity of DNA methyltransferase DNMT1, leading to chromatin opening and activation of Wnt target genes [8]. The Porcupine inhibitor LGK974, which blocks Wnt ligand secretion, effectively inhibits the self-renewal of LGR5-positive cancer stem cells and has now entered clinical trials [9].
3.2 Cross-Regulation with Other Signaling Pathways
In addition to the canonical Wnt pathway, LGR5 interacts with multiple signaling networks. In lipid metabolism, LGR5 affects PPARγ expression, regulating adipocyte differentiation and energy metabolism balance [2]. Under oxidative stress, LGR5 can influence the expression of antioxidant enzymes by regulating the nuclear translocation of FoxO1, maintaining genomic stability.
Furthermore, LGR5 also exhibits cross-regulatory interactions with the Notch pathway, TGF-β signaling, and the non-canonical Wnt/PCP pathway. Under viral infection, changes in LGR5 expression can interfere with the transcriptional activity of the Notch target gene Hes1, affecting cell differentiation processes. In colorectal cancer, high LGR5 expression enhances Wnt signaling via RSPO and silences tumor suppressor genes through epigenetic mechanisms, forming a positive feedback loop [7][8]. The interaction between LGR5 and the TGF-β/Smad pathway can promote epithelial-mesenchymal transition, enhancing tumor invasiveness. These cross-regulatory mechanisms highlight the multifunctional role of LGR5 as a signaling hub.
4. Association Between LGR5 Signaling Abnormalities and Disease
Abnormal LGR5 signaling is closely associated with various diseases. In colorectal cancer, LGR5 promotes the maintenance of tumor stemness through overactivation of the Wnt/β-catenin pathway, and its high expression is significantly correlated with tumor recurrence, metastasis, and drug resistance [7]. LGR5-positive cells drive cell cycle abnormalities and epithelial-mesenchymal transition by stabilizing β-catenin nuclear translocation and upregulating genes like c-Myc and Cyclin D1 [5]. Epigenetic analysis indicates that demethylation of the LGR5 promoter is an important reason for its high expression [8].
In metabolic diseases, LGR5 cross-regulates with lipid metabolism pathways; in patients with obstructive sleep apnea (OSA), LGR5 is significantly correlated with lipid metabolism disorders, potentially affecting the oxidative stress response via FoxO signaling [2]. In the cardiovascular system, abnormal LGR5 expression affects angiogenesis and endothelial function, suggesting its potential as a target in various chronic diseases.
5. Research Progress on LGR5 in Cancer and Other Diseases
5.1 Role in Colorectal Cancer
As a cancer stem cell marker, LGR5 promotes tumor cell proliferation, migration, and chemotherapy resistance in colorectal cancer by activating Wnt signaling [7]. The LGR5–RSPO complex inhibits RNF43/ZNRF3, prolonging Wnt signal activation and inducing β-catenin nuclear translocation and downstream c-Myc and Cyclin D1 transcription [5].
the colorectal cancer microenvironment, LGR5 synergistically regulates metabolic reprogramming with the PI3K/AKT pathway, enhancing fatty acid uptake to meet energy demands [2]. LGR5+ cells are often enriched at the invasive front of tumors and co-express EMT markers like N-cadherin and vimentin, driving metastasis [11]. Animal experiments show that LGR5 knockout significantly inhibits tumor growth and metastasis and enhances sensitivity to 5-FU [7].
Targeted therapeutic strategies include monoclonal antibodies blocking the RSPO/LGR5 interaction, small molecule inhibitors targeting downstream Wnt components, and epigenetic drugs. Anti-LGR5 antibody-drug conjugates (ADCs) can specifically eliminate cancer stem cells and significantly enhance immune response when combined with PD-1 inhibitors [5]. Furthermore, HDAC inhibitors downregulate LGR5 expression through chromatin remodeling, reversing the drug-resistant phenotype [10]. LGR5-targeted CAR-T therapy has entered Phase I clinical trials, with the main challenge being avoiding toxicity to normal intestinal stem cells [11].
5.2 Role in Other Diseases
In obstructive sleep apnea (OSA), LGR5 is involved in the disease process by regulating lipid metabolism. LGR5 expression is elevated in peripheral blood mononuclear cells of OSA patients and positively correlates with triglyceride and LDL levels [2]. Single-cell RNA sequencing found enrichment of LGR5+ cells in the respiratory tract tissues of OSA patients, exhibiting enhanced inflammatory and oxidative stress responses [12].
In liver diseases, abnormal proliferation of LGR5-positive liver progenitor cells is closely related to liver fibrosis and hepatocellular carcinoma. A zebrafish liver tumor model showed that LGR5 activates Wnt signaling, promoting hepatic stellate cell activation and collagen deposition [13]. Clinical sample analysis revealed that LGR5 is 3-5 times higher in cirrhotic liver tissue compared to normal liver and positively correlates with disease severity [13][14].
Additionally, depletion of LGR5-positive stem cells is closely associated with aging. LGR5 expression decreases in aged intestinal organoids accompanied by reduced proliferative capacity; in Alzheimer's disease models, LGR5 downregulation leads to impaired neuroregeneration and cognitive decline [12]; skin research indicates that a reduction in LGR5-positive hair follicle stem cells is directly related to skin aging characteristics.
6. Progress in Drug Development Targeting LGR5
Currently, drug development targeting LGR5 primarily focuses on antibody-based drugs (bispecific antibodies, ADCs) and cell therapies (CAR-T). The main indication is colorectal cancer. The highest development stage is Phase 3, represented by Petosemtamab, which is also the key asset involved in this acquisition. Other pipelines in research are summarized in the table below:
| Drug |
Mechanism of Action |
Drug Type |
Indications Under Investigation |
R&D Institutions |
Highest Phase |
| Petosemtamab |
EGFR antagonist | LGR5 antagonist |
Bispecific Antibody |
Recurrent Head & Neck Squamous Cell Carcinoma | Metastatic Head & Neck Squamous Cell Carcinoma | Advanced Malignant Solid Tumor | Esophageal Cancer | Gastroesophageal Junction Cancer | Metastatic Colorectal Cancer | Non-Small Cell Lung Cancer |
Merus NV |
Phase 3 |
| CNA-3103 |
LGR5 antagonist | Immunocytotoxicity | T-cell Stimulator |
Autologous CAR-T |
Metastatic Colorectal Cancer | Colorectal Cancer | Hematologic Tumor | Ovarian Cancer |
Carina Biotech Ltd. | Bionomics Ltd. |
Phase 1/2 |
| R462-CPT2 |
LGR4 inhibitor | LGR5 antagonist | LGR6 antagonist |
Peptide-Drug Conjugate (PDC) |
Colorectal Cancer |
The University of Texas Health Science Center at Houston |
Preclinical |
| LGR5-CAR-T (Adelaide) |
LGR5 antagonist |
CAR-T |
Colorectal Cancer |
University of Adelaide | Carina Biotech Ltd. |
Preclinical |
| 8F2-SG3199 |
DNA inhibitor | LGR5 antagonist |
ADC |
Neuroblastoma |
UTHealth Houston | The University of Texas Health Science Center at Houston |
Preclinical |
| R20-SG3199 |
DNA inhibitor | LGR5 antagonist |
ADC |
Neuroblastoma |
The University of Texas Health Science Center at Houston |
Preclinical |
| LGR5-ADC (University of Texas Health Science Center) |
LGR5 antagonist |
ADC |
Colorectal Cancer |
The University of Texas Health Science Center at Houston |
Preclinical |
(Data as of October 25, 2025, source: synapse)
7. CUSABIO LGR5 Research-Related Products
FLT1, as a key factor in angiogenesis and immune regulation, has become a research focus in various diseases. Its aberrant expression in CMS, PAH, OA, tumors, and other diseases suggests it is not only a potential diagnostic marker but also an important target for therapeutic intervention.
CUSABIO has developed a variety of FLT1-related research products, including recombinant proteins, antibodies, and ELISA kits, to assist your research into FLT1 mechanisms and drug development.
References
[1] Kaavya Krishna Kumar1,Antony W Burgess,Jacqueline M Gulbis. Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells[J]. Protein Sci, 2014, 5.
[2] Peng Lü, Xiaodi Wang, Dan Bing. Identification and Validation of Prognostic Factors of Lipid Metabolism in Obstructive Sleep Apnea[J]. Frontiers in Genetics, 2021, 12.
[3]Sang-Myung Jung, Seonghun Kim. In vitro Models of the Small Intestine for Studying Intestinal Diseases[J]. Frontiers in Microbiology, 2022, 12.
[4] Hong Ouyang, Jeffrey L. Goldberg, Shuyi Chen, Wei Li, Guo-Tong Xu, Wei Li, Kang Zhang, Robert B. Nussenblatt, Wei Li, Ting Xie, Chi‐Chao Chan, Donald J. Zack. Ocular Stem Cell Research from Basic Science to Clinical Application: A Report from Zhongshan Ophthalmic Center Ocular Stem Cell Symposium[J]. International Journal of Molecular Sciences, 2016, 17(3): 415-415.
[5] Jia Bian, Marius Dannappel, Chunhua Wan, Ron Firestein. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer[J]. Cells, 2020, 9(9): 2125-2125.
[6] Clémence Bonnet, Anvi Brahmbhatt, Sophie X. Deng, Jie Zheng. Wnt signaling activation: targets and therapeutic opportunities for stem cell therapy and regenerative medicine[J]. RSC Chemical Biology, 2021, 2(4): 1144-1157.
[7] Hui Zhao, Tianqi Ming, Shun Tang, Shan Ren, Han Yang, Maolun Liu, Tao Qiu, Haibo Xu. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target[J]. Molecular Cancer, 2022, 21(1).
[8] Ankita Sharma, Rafeeq Mir, Sanjeev Galande. Epigenetic Regulation of the Wnt/β-Catenin Signaling Pathway in Cancer[J]. Frontiers in Genetics, 2021, 12.
[9] Dario Zimmerli, George Hausmann, Claudio Cantù, Konrad Basler. Pharmacological interventions in the Wnt pathway: inhibition of Wnt secretion versus disrupting the protein–protein interfaces of nuclear factors[J]. British Journal of Pharmacology, 2017, 174(24): 4600-4610.
[10] Bing Liang, Yanhong Wang, Jiazhen Xu, Yingchun Shao, Dongming Xing. Unlocking the potential of targeting histone-modifying enzymes for treating IBD and CRC[J]. Clinical Epigenetics, 2023, 15(1).
[11] Yagmur Azbazdar, Mustafa Karabicici, Esra Erdal, Günes Özhan. Regulation of Wnt Signaling Pathways at the Plasma Membrane and Their Misregulation in Cancer[J]. Frontiers in Cell and Developmental Biology, 2021, 9.
[12] Hina Agraval, Hong Wei Chu. Lung Organoids in Smoking Research: Current Advances and Future Promises[J]. Biomolecules, 2022, 12(10): 1463-1463.
[13] Qizhuan Lin, Libo Jin, Renyi Peng. New Progress in Zebrafish Liver Tumor Models: Techniques and Applications in Hepatocellular Carcinoma Research[J]. International Journal of Molecular Sciences, 2025, 26(2): 780-780.
[14] Yaqi Li, Peiyuan Tang, Sanjun Cai, Junjie Peng, Guoqiang Hua. Organoid based personalized medicine: from bench to bedside[J]. Cell Regeneration, 2020, 9(1)
CUSABIO team. The "Achilles' Heel" of Cancer Stem Cells: LGR5 Becomes Focus of $8 Billion Acquisition. https://www.cusabio.com/c-21265.html

-WB.jpg)
-AC1.jpg)



Comments
Leave a Comment