[1] Weixiang Zhan, F. Bai, Yue Cai, Jianwei Zhang, Ge Qin, Yuqian Xie, Yanhong Deng.(2023). Tumor stroma Siglec15 expression is a poor prognosis predictor in colon adenocarcinoma.
[2] Jiao Hu, Anze Yu, Belaydi Othmane, D. Qiu, Huihuang Li, Chao Li, Peihua Liu, Wenbiao Ren, Minfeng Chen, G. Gong, Xi Guo, Huihui Zhang, Jinbo Chen, X. Zu.(2021). Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer.
[3] Huan Lai, Yiyang Liu, Yan Gong, Chuanyu Zong, Wei Zeng, Honglei Chen.(2024). Expression of SIGLEC15 correlates with tumor immune infiltration, molecular subtypes, and breast cancer progression.
[4] Zheng Chen, Mincheng Yu, Bo Zhang, Lei Jin, Qiang Yu, Shuang-Hu Liu, Binghai Zhou, Jiuliang Yan, Wentao Zhang, Xiaoqiang Li, Yongfeng Xu, Yongsheng Xiao, Jian Zhou, Jia Fan, M. Hung, Qinghai Ye, Hui Li, Lei Guo.(2024). SIGLEC15, negatively correlated with PD-L1 in HCC, could induce CD8+ T cell apoptosis to promote immune evasion.
[5] Haifeng Liang, Lei Zhou, Z. Hu, Yuxiang Ge, Tai-Wei Zhang, Qing Chen, Ben Wang, Shunyi Lu, Wang Ding, J. Dong, Fengfeng Xue, Libo Jiang.(2022). Siglec15 Checkpoint Blockade for Simultaneous Immunochemotherapy and Osteolysis Inhibition in Lung Adenocarcinoma Spinal Metastasis via a Hollow Nanoplatform.
[6] Xiaofeng Hou, Chao Chen, X. Lan, Xiao-dong He.(2022). Unveiling the molecular features, relevant immune and clinical characteristics of SIGLEC15 in thyroid cancer.
[7] Claire E Pillsbury, Jodi Dougan, Jennifer L. Rabe, Jairo A Fonseca, Chengjing Zhou, Alyssa N Evans, Hasan Abukharma, Ona Ichoku, Gloria Gonzalez-Flamenco, Sunita I Park, A. Aljudi, D. DeRyckere, S. Castellino, S. Rafiq, S. Langermann, Linda N. Liu, C. Henry, Christopher C Porter.(2023). Siglec-15 Promotes Evasion of Adaptive Immunity in B-cell Acute Lymphoblastic Leukemia.
[8] Jinchao Wang, Lin-gen Xu, Qian Ding, Xiaoru Li, Kai Wang, Shangchen Xu, Bin Liu.(2023). Siglec15 is a prognostic indicator and a potential tumor-related macrophage regulator that is involved in the suppressive immunomicroenvironment in gliomas.
[9] Juan Wang, Baosheng Li.(2025). Siglec15 as a potential therapeutic target for the treatment of fibrosarcoma.
[10] D. Deng, Jiatong Xiao, Jinhui Liu, Huihuang Li, Minghui Hu, Bohan Zhou, Haisu Liang, Benyi Fan, Jinbo Chen, Xiaogen Kuang, Zhenyu Nie, Jiao Hu, Xiongbing Zu.(2024). Evasion of immunosurveillance by the upregulation of Siglec15 in bladder cancer.
[11] Tianjiao Li, Kaifeng Jin, Hao Li, L. Ye, Pengcheng Li, Bruce Jiang, Xuan Lin, Zhen-Yu Liao, Hui-Ru Zhang, Saimeng Shi, Mengxiong Lin, Qinglin Fei, Zhichun Xiao, Hua-Xiang Xu, Liang Liu, Xianjun Yu, Wei Wu.(2022). SIGLEC15 amplifies immunosuppressive properties of tumor-associated macrophages in pancreatic cancer.
[12] R. Takamiya, K. Ohtsubo, S. Takamatsu, N. Taniguchi, T. Angata.(2013). The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway.
[13] Xianghong Chen, E. Eksioglu, J. Carter, Nicole R. Fortenbery, Sarah S. Donatelli, Jun-min Zhou, Jinhong Liu, Li-li Yang, D. Gilvary, J. Djeu, Sheng Wei.(2015). Inactivation of DAP12 in PMN Inhibits TREM1-Mediated Activation in Rheumatoid Arthritis.
[14] A. Zarbock, C. Abram, M. Hundt, A. Altman, C. Lowell, K. Ley.(2008). PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcRγ to induce slow leukocyte rolling.
[15]Frank Fasbender, M. Claus, S. Wingert, M. Sandusky, C. Watzl.(2017). Differential Requirements for Src-Family Kinases in SYK or ZAP70-Mediated SLP-76 Phosphorylation in Lymphocytes.
[16] Sijing Huang, Zhi Ji, Jinqiang Xu, Yue-ning Yang, Bingrui Wu, Qihang Chen, Shuting Geng, Yu Si, Jiayue Chen, Yuanyan Wei, Cong Wang, Zhilong Ai, Jianhai Jiang.(2023). Siglec15 promotes the migration of thyroid carcinoma cells by enhancing the EGFR protein stability.
[17] T. Ochi, Yuko Matsuoka, Tatsuya Konishi, M. Maruta, Y. Miyazaki, K. Tanimoto, Masaki Yasukawa, Masakatsu Yamashita, Katsuto Takenaka.(2023). Development of a New CAR-T-Cell Therapy Targeting an Immune Checkpoint for Effective Treatment of Solid and Liquid Tumors.
[18] Yutong Wu, Hongbo Ai, Yuhang Xi, Jiulin Tan, Ying Qu, Jianzhong Xu, F. Luo, C. Dou.(2023). Osteoclast-derived apoptotic bodies inhibit naive CD8+ T cell activation via Siglec15, promoting breast cancer secondary metastasis.

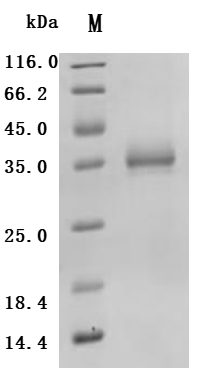
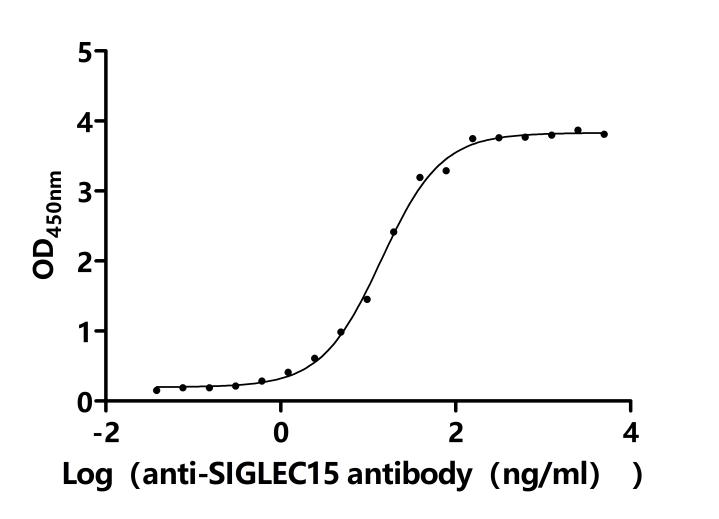
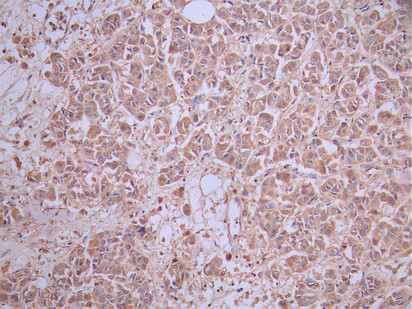


Comments
Leave a Comment