TNFRSF13B (TACI), a molecule playing a central role in immune regulation, has become a new focal point for cross-domain breakthroughs in tackling tumors and autoimmune diseases due to its functional diversity. Current research breakthroughs are intensively emerging in innovative dual-target strategies aimed at achieving a "1+1>2" therapeutic effect. This article will systematically outline the latest research landscape of this potential target, starting from its signaling pathways and disease mechanisms.
1. Research Background of TNFRSF13B (TACI)
TNFRSF13B (Tumor Necrosis Factor Receptor Superfamily Member 13B), also known as TACI (Transmembrane Activator and CAML Interactor), is an important member of the TNF receptor superfamily. This gene is located on human chromosome 17p11.2, and its encoded TACI protein plays a core role in immune regulation, B cell homeostasis maintenance, and various disease processes. TACI can bind to APRIL (A Proliferation-Inducing Ligand) and BAFF (B Cell Activating Factor), regulating the proliferation, differentiation, and survival of B cells, and is crucial for maintaining humoral immune homeostasis [2,4].
Under normal conditions, TACI activation promotes B cell differentiation into plasma cells, facilitating immunoglobulin class switching and antibody production [2,6]. However, aberrant expression or mutation of TNFRSF13B can disrupt immune balance and is associated with various diseases, including Chronic Lymphocytic Leukemia (CLL), melanoma, Lupus Nephritis (LN), Multisystem Inflammatory Syndrome in Children (MIS-C), and Common Variable Immunodeficiency Disease (CVID) [1,4,6,7,8].
2. Mechanism of Action of TNFRSF13B (TACI)
2.1 Immunoregulatory Mechanism
The primary function of TNFRSF13B is to regulate B cell immune responses by binding to ligands such as APRIL and BAFF. In the inflammatory microenvironment, interaction between membrane-bound APRIL and TACI on the B cell surface can induce macrophages to release inflammatory mediators like IL-8 and MMP-9, promoting chronic inflammation [2]. The co-expression levels of TNFRSF13B and APRIL in the synovial tissue of rheumatoid arthritis patients positively correlate with the degree of inflammation, suggesting a key role for the TACI-APRIL axis in the persistence of chronic inflammation [2].
In normal immune processes, TACI activation promotes B cell maturation and antibody production. When TNFRSF13B mutations occur (e.g., A181E), its ligand-binding capacity decreases, leading to impaired B cell activation, reduced immunoglobulin production, and consequently causing Common Variable Immunodeficiency Disease (CVID). Furthermore, rare pathogenic variants of TNFRSF13B have been detected in MIS-C patients; these variants disrupt immune homeostasis and exacerbate inflammatory responses [8].
2.2 Tumor Regulatory Mechanism
In tumors, TNFRSF13B can exert effects by regulating cell survival, proliferation, and the tumor immune microenvironment. During the transformation of Chronic Lymphocytic Leukemia (CLL) to Richter Syndrome (RS), TNFRSF13B expression is upregulated. Binding with APRIL and BAFF in the tumor microenvironment activates the NF-κB pathway, promoting tumor cell proliferation [6]. In melanoma, its high expression promotes Regulatory T cell (Treg) enrichment and enhances immunosuppressive function via the JAK-STAT pathway, inhibiting effector T cell activity, thereby facilitating tumor immune escape [7]. Additionally, in breast cancer, TNFRSF13B expression is negatively correlated with mammographic density and cancer risk [3,5].
3. TNFRSF13B (TACI) Related Signaling Pathways
3.1 NF-κB Signaling Pathway
NF-κB is a core downstream pathway of TNFRSF13B. Upon ligand binding, TACI activates the IKK complex, induces IκB degradation, and releases NF-κB into the nucleus, regulating the expression of target genes such as IL-6 and Bcl-2, promoting cell survival and inflammatory responses [2,6]. This pathway is persistently active in CLL/RS and Lupus Nephritis (LN), driving disease progression [4,6].
3.2 JAK-STAT Signaling Pathway
TNFRSF13B activates the JAK3-STAT5 pathway, promoting Treg differentiation and immunosuppressive function. In melanoma, high expression of TNFRSF13B is significantly correlated with STAT5 phosphorylation levels, and STAT5 inhibition can restore effector T cell activity [7]. Furthermore, TNFRSF13B variants in MIS-C may lead to abnormal immune cell activation by affecting the JAK-STAT pathway [8].
3.3 Other Pathways
TACI also promotes B cell metabolism and survival by activating the PI3K-AKT pathway, enhancing drug resistance in CLL cells [2,6]; it also synergizes with the TGF-β pathway to promote tumor Epithelial-Mesenchymal Transition (EMT), exacerbating tumor metastasis [5,7].
4. TNFRSF13B (TACI) Related Diseases
4.1 Hematologic Diseases
4.1.1 Chronic Lymphocytic Leukemia (CLL) and Richter Syndrome (RS)
CLL is a common mature B-cell malignancy, with approximately 2-10% of patients eventually progressing to RS. Studies show that TNFRSF13B expression is significantly upregulated in tumor cells of RS patients, and its level positively correlates with NF-κB pathway activity [6]. TACI binds to APRIL and BAFF secreted by monocytes, promoting tumor cell survival and inflammatory factor release, thereby accelerating the transformation of CLL to RS [6]. Moreover, patients with high TNFRSF13B expression have a poorer prognosis and significantly shorter survival, suggesting its potential as a biomarker.
4.1.2 Multisystem Inflammatory Syndrome in Children (MIS-C)
MIS-C is a severe inflammatory reaction syndrome following SARS-CoV-2 infection. Approximately 6.7% of patients harbor TNFRSF13B pathogenic variants, primarily frameshift mutations (e.g., c.20-idup) [8]. These variants impair TACI structure and signal transduction function, disrupting B cell regulation and thereby triggering abnormal inflammatory responses and multi-organ damage. Patients carrying these mutations are more prone to shock and cardiac dysfunction, indicating that TNFRSF13B is an important molecular marker affecting disease severity [8].
4.2 Autoimmune Diseases
4.2.1 Lupus Nephritis (LN)
LN is a severe complication of Systemic Lupus Erythematosus. Studies show that TNFRSF13B mRNA can be detected in patient urinary sediment, and its expression frequency correlates with LN type and disease activity [4]. The detection rate is significantly higher in Type III/IV LN compared to Type V (42.85% vs. 0%). Activation of the TACI-BAFF/APRIL axis triggers the NF-κB pathway, inducing the expression of inflammatory factors IL-6, TNF-α, and chemokine CCL21, promoting immune cell infiltration and immune complex deposition [4]. Furthermore, urinary TACI expression levels closely correlate with the SLEDAI-2K index and complement C4 levels, suggesting its potential value as a non-invasive biomarker for LN [4].
4.3 Malignant Tumors
4.3.1 Melanoma
Melanoma is the most aggressive skin cancer. TNFRSF13B is highly expressed in melanoma tissue and is closely associated with lymph node metastasis [7]. TACI can upregulate FOXP3 expression via the JAK-STAT pathway, promoting Treg proliferation and enhancing the immunosuppressive environment [7]. Simultaneously, it synergizes with immunosuppressive molecules like LAG3 and NT5E, further weakening anti-tumor immune responses. Patients with high TNFRSF13B expression have significantly shorter overall survival and progression-free survival [7].
4.3.2 Breast Cancer
Research indicates that TNFRSF13B expression is negatively correlated with mammographic density, and increased BMI is significantly associated with decreased gene expression (an 8.5% decrease per 10 kg/m² increase) [3,5]. Since mammographic density is an independent risk factor for breast cancer, TNFRSF13B may influence cancer risk by regulating the proliferation and differentiation of mammary epithelial cells [5]. Additionally, its interaction with molecules such as ESR1 and RANK may affect the estrogen pathway and breast tissue homeostasis, further increasing the probability of tumorigenesis.
4.4 Immunodeficiency Diseases
Common Variable Immunodeficiency Disease (CVID) is a primary immunodeficiency characterized by B cell functional defects and antibody production impairment. Approximately 10%-20% of CVID patients carry TNFRSF13B pathogenic mutations (e.g., A181E, C104R), which weaken TACI's binding capacity to BAFF/APRIL [1]. Functionally, these mutations inhibit B cell activation, immunoglobulin class switching, and plasma cell generation, leading to recurrent infections and autoimmune complications in patients. Patients with mutations are more likely to develop severe diseases such as chronic lung disease and lymphoma [1].
5. Research Progress on TNFRSF13B (TACI) Targeted Drugs
Current drug development targeting TNFRSF13B (TACI) shows clear strategic trends. To overcome the heterogeneity of complex diseases and improve efficacy, "dual-target" and even "multi-target" strategies have become mainstream in this field. This strategy aims to simultaneously block multiple pathogenic pathways or address antigen escape in cancer therapy. In oncology, BCMA/TACI dual-target CAR-T/CAR-NK cell therapies are being developed for Multiple Myeloma to reduce the risk of relapse due to antigen loss after single-target therapy. In autoimmunity, companies like Hengrui Medicine are actively developing bispecific proteins fusing TACI with targets like IL-23, IFNAR-1, and BCMA, aiming to synergistically inhibit multiple inflammatory pathways with a single molecule for treating complex diseases like Systemic Lupus Erythematosus. Although most projects are still in the preclinical stage, the technological approaches are diversifying. The pipeline under investigation is summarized in the table below:
| Drug |
Mechanism of Action |
Drug Type |
Indications Under Investigation |
Research Institution |
Highest Development Phase |
| LMY-920 |
BAFF-R inhibitor | BCMA inhibitor | TACI modulator |
Autologous CAR-T |
Refractory Chronic Lymphocytic Leukemia | Relapsed Chronic Lymphocytic Leukemia, etc. |
Luminary Therapeutics, Inc. | The Case Comprehensive Cancer Center | Case Western Reserve University |
Phase 1 |
| BCMA, TACI Dual-Targeting CAR-T (Massachusetts General Hospital) |
BCMA modulator | TACI modulator |
CAR-T |
Multiple Myeloma |
The Massachusetts General Hospital |
Preclinical |
| IL-12p40/TACI Antibody Fusion Protein (Jiangsu Hengrui Pharmaceuticals) |
IL-12R stimulator | TNFRSF13B inhibitor |
Antibody Fusion Protein |
Systemic Lupus Erythematosus |
Jiangsu Hengrui Pharmaceuticals Co., Ltd. |
Preclinical |
| Anti-IL23 Antibody-TACI Fusion Protein (Hengrui Medicine) |
IL-23 modulator | TACI modulator |
Antibody Fusion Protein |
Systemic Lupus Erythematosus |
Jiangsu Hengrui Pharmaceuticals Group Co., Ltd. |
Preclinical |
| Anifrolumab-TACI Fusion Protein (Hengrui Medicine) |
IFNAR-1 modulator | TACI modulator |
Antibody Fusion Protein |
Autoimmune Disease |
Jiangsu Hengrui Pharmaceuticals Group Co., Ltd. |
Preclinical |
| TST-008 |
MASP2 inhibitor | TNFRSF13B inhibitor |
Bispecific Antibody |
Kidney Disease | Systemic Lupus Erythematosus |
Transcenta Holding Limited |
Preclinical |
| iPSC CAR-NK TACI/BCMA (Kite Pharma) |
BCMA modulator | TACI modulator |
CAR-NK |
Multiple Myeloma |
Kite Pharma, Inc. |
Preclinical |
| EPC-004 |
BCMA inhibitor | TACI modulator | Immunocytotoxicity | T lymphocyte replacement |
CAR-NK |
Multiple Myeloma |
Elpis Biopharmaceuticals Corp. |
Preclinical |
| Anti-IFNAR1/ TACI fusion protein (Jiangsu Hengrui Pharmaceutical) |
IFNAR-1 antagonist | TNFRSF13B inhibitor |
Fusion Protein |
Autoimmune Disease | Inflammation |
Jiangsu Hengrui Pharmaceuticals Group Co., Ltd. |
Preclinical |
| Anifrolumab-TACI-BCMA Fusion Protein (Hengrui Medicine) |
BCMA modulator | IFNAR-1 modulator | TACI modulator |
Antibody Fusion Protein |
Autoimmune Disease |
Jiangsu Hengrui Pharmaceuticals Group Co., Ltd. |
Preclinical |
| HLX318 |
BAFF agonist | BCMA inhibitor | TACI modulator |
Fusion Protein |
Autoimmune Disease |
Shanghai Henlius Biotech, Inc. |
Preclinical |
| SIM0380 |
IL-17 inhibitor | TACI modulator |
Antibody Fusion Protein |
Autoimmune Disease |
Simcere Pharmaceutical Group Ltd. |
Preclinical |
| TACI-Fc Fusion Protein (Hengrui Medicine) |
TNFRSF13B inhibitor |
Fc Fusion Protein |
Systemic Lupus Erythematosus |
Jiangsu Hengrui Pharmaceuticals Co., Ltd. |
Preclinical |
| TACI-targeting CAR-T (Massachusetts General Hospital) |
TACI modulator |
CAR-T |
Multiple Myeloma |
The Massachusetts General Hospital |
Preclinical |
| BAFF CAR-T cells (Case Western Reserve University) |
BAFF-R inhibitor | BCMA inhibitor | TNFRSF13B inhibitor |
CAR-T |
Hairy Cell Leukemia |
Case Western Reserve University School of Medicine |
Preclinical |
(Data as of October 25, 2025, Source: synapse)
6. TNFRSF13B (TACI) Research Tools
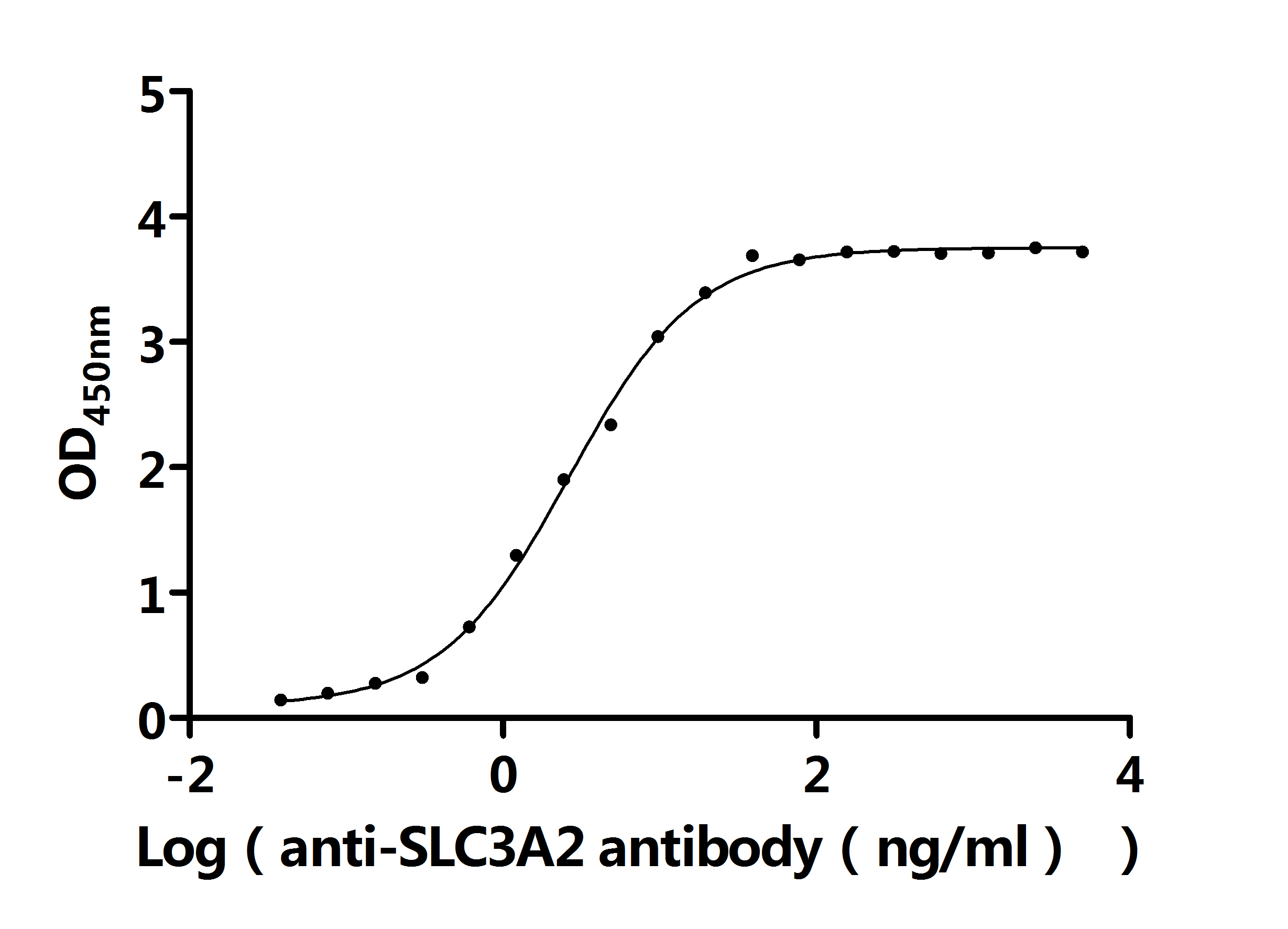
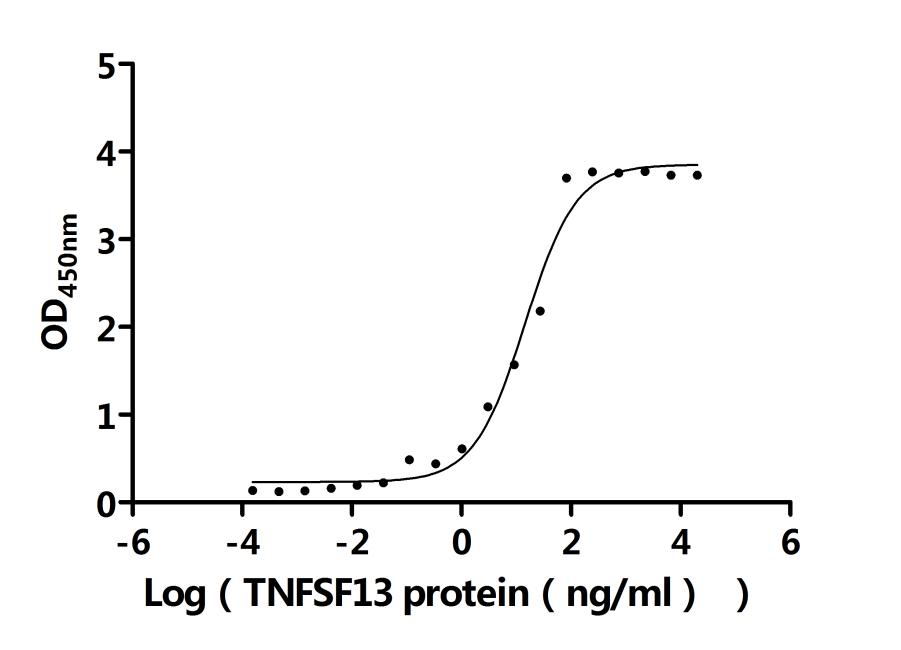
TNFRSF13B is a key member of the TNF receptor superfamily and plays an important role in immune regulation and disease pathogenesis. It regulates immune homeostasis through pathways such as NF-κB and JAK-STAT, but its aberrant expression or mutations are closely associated with various diseases. CUSABIO provides TNFRSF13B (TACI) recombinant proteins, antibodies, and ELISA kits to assist you in developing drugs specifically targeting TNFRSF13B and exploring its application potential in the treatment of tumors and autoimmune diseases.
References
[1] Liquidano-Perez E, et al. Case Report: Hydroa vacciniforme-like lymphoproliferative disorder, an EBV-associated disease, successfully treated with hematopoietic stem cell transplantation. Front Immunol. 2025;16:1511385.
[2] Lee S-M, et al. Cell to Cell Interaction Can Activate Membrane-bound APRIL Which Are Expressed on Inflammatory Macrophages. Immune Netw. 2010;10 (5):173-180.
[3] Han Y, et al. Changes in adiposity over the life course and gene expression in postmenopausal women. Cancer Med. 2022;11:2699-2710.
[4] Aguirre-Valencia D, et al. Expression of BAFF, APRIL, and cognate receptor genes in lupus nephritis and potential use as urinary biomarkers. J Transl Autoimmun. 2020;3:100027.
[5] Mintz R, et al. Hormone and receptor activator of NF-κB (RANK) pathway gene expression in plasma and mammographic breast density in postmenopausal women. Breast Cancer Res. 2022;24:28.
[6] Rohan P, et al. NF-ΚB Activation as a Key Driver in Chronic Lymphocytic Leukemia Evolution to Richter's Syndrome: Unraveling the Influence of Immune Microenvironment Dynamics. Genes. 2024;15:1434.
[7] Zhang C, et al. Pivotal factors associated with the immunosuppressive tumor microenvironment and melanoma metastasis. Cancer Med. 2021;10:4710-4720.
[8] Reis BCSD, et al. Rare genetic variants involved in multisystem inflammatory syndrome in children: a multicenter Brazilian cohort study. Front Cell Infect Microbiol. 2023;13:1182257.
CUSABIO team. Targeting TNFRSF13B: Dual-Target Strategies Usher in New Focus for Treating Tumors and Autoimmune Diseases. https://www.cusabio.com/c-21269.html



Comments
Leave a Comment