Function
Catalyzes the ATP-dependent ligation of glycine to the 3'-end of its cognate tRNA, via the formation of an aminoacyl-adenylate intermediate (Gly-AMP). Also produces diadenosine tetraphosphate (Ap4A), a universal pleiotropic signaling molecule needed for cell regulation pathways, by direct condensation of 2 ATPs. Thereby, may play a special role in Ap4A homeostasis.
Gene References into Functions
- In support of GARS variant pathogenicity, our patient shows striking phenotypic overlap with other patients having ARS-related recessive diseases; this observation is consistent with the essential function of GARS in both cellular locations. In summary, our clinical, genetic, and functional analyses expand the phenotypic spectrum associated with GARS variants PMID: 28675565
- In this Chinese Han population a novel Charcot-Marie-Tooth disease-associated gene mutations of GARS (c.794C>T) was discovered. PMID: 27862672
- At the active site, a glycyl-AMP molecule is synthesized and is waiting for the transfer of the glycyl moiety to occur. PMID: 27261259
- GlyRS functions as a chaperone that critically supports neddylation. PMID: 27348078
- Data indicate that dimerization is required for the dominant neurotoxicity of disease-associated GARS mutations and provide a rapid, tractable model for studying newly identified GARS variants for a role in human disease. PMID: 27008886
- one of the mRNAs isoforms tightly controls expression and localization of human GARS. PMID: 26327585
- This study reports two crystal structures of human GlyRS variants, in the free form and in complex with tRNA(Gly) respectively, and reveal new aspects of the glycylation mechanism. PMID: 26797133
- GARS mutations are an uncommon cause of Charcot-Marie-Tooth Disease (CMT) in Taiwan. The p.Asp146Tyr and p.Met238Arg mutations are associated with early-onset axonal CMT. PMID: 26244500
- Our findings suggest that mutant GlyRS gains access to ectopic sub-compartments of the motor neuron, providing a possible explanation for the selective neuropathology caused by mutations in a widely expressed gene. PMID: 25972375
- Expression of three CMT-mutant GARS proteins in Drosophila induces defects in motor performance and motor and sensory neuron morphology, and shortens lifespan. PMID: 26138142
- The c.999G>T mutation is a novel mutation of the glycyl-tRNA synthetase gene that has not been previously reported. The phenotype of this family is Charcot-Marie-Tooth disease type 2D, which is first reported in Chinese population. PMID: 26000875
- we propose that the disease-causing L129P mutant of glycyl-tRNA synthetase is linked to a distribution defect in peripheral nerves in vivo. PMID: 25218976
- our data indicate that impaired function is a key component of GARS-mediated CMT disease and emphasize the need for careful genetic and functional evaluation before implicating a variant in disease onset. PMID: 25168514
- This study presents genetic evidence for common mutant-specific interactions between two CMT-associated aminoacyl-tRNA synthetases, lending support for a shared mechanism responsible for the synthetase-induced peripheral neuropathies. PMID: 24807208
- We developed an ELISA to detect anti-glycyl-tRNA synthetase by using recombinant protein PMID: 24508626
- Report crystal structures of wild type and mutant GlyRS in complex with tRNA and with small substrates and describe the molecular details of enzymatic recognition of the key tRNA identity elements in the acceptor stem and the anticodon loop. PMID: 24898252
- we believe that these two novel GARS mutations are the underlying causes of the distal hereditary motor neuropathy type V phenotype PMID: 23279345
- GRS bound to different ERK-activated tumor cells, and released phosphatase 2A (PP2A) from CDH6. PMID: 22345558
- missense mutations of Gars may cause some loss of function, the dominant neuropathy phenotype observed in mice is caused by a dose-dependent gain of function that is not mitigated by over-expression of functional wild-type protein. PMID: 22144914
- No pathogenic mutations were found, excluding the role of GARS gene as a possible factor in the aetiology of Hirayama disease in this cohort PMID: 19412816
- GARS mutation is a rare cause of Charcot-Marie-Tooth neuropathy among Japanese patients. PMID: 19329989
- Four disease-associated missense mutations in the glycyl tRNA synthetase gene in families with Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V PMID: 12690580
- A novel heterozygous missense GARS gene mutation (D500N) was identified in members of a family affected byCharcot-Marie-Tooth type 2D. PMID: 16534118
- We screened 100 patients with inherited and sporadic lower motor neuron degeneration and identified three novel missense mutations in the glycyl-tRNA synthetase (GARS) gene. PMID: 17101916
- The crystal belonged to space group P4(3)2(1)2 or its enantiomorphic space group P4(1)2(1)2, & diffracted X-rays to 3.0 A resolution. The asymmetric unit contained 1 GlyRS molecule & had a solvent content of 69%. PMID: 17142907
- Crystal structure of human wildtype and S581L-mutant glycyl-tRNA synthetase. PMID: 17544401
- The structure of wild type and Charcot-Marie-Tooth-causing mutant of homodimeric GlyRS are reported. PMID: 17545306
- Charcot-Marie-Tooth (CMT) disease-causing mutations of glycine-tRNA synthetase share a common defect in localization which may be connected to a change in surfaces at the dimer interface, and may cause a dominant axonal form of CMT (type 2D). PMID: 17595294
- we present a comparison between the crystal structures of the eubacterial Escherichia coli and the human tRNA(Gly) acceptor stem microhelices and their surrounding hydration patterns. PMID: 18275849
- human glycyl-tRNA synthetase has a role in Ap4A homeostasis PMID: 19710017
Show More
Hide All
Involvement in disease
Charcot-Marie-Tooth disease 2D (CMT2D); Neuronopathy, distal hereditary motor, 5A (HMN5A)
Subcellular Location
Cytoplasm. Cell projection, axon. Secreted. Secreted, extracellular exosome.; [Isoform 1]: Mitochondrion. Cytoplasm.; [Isoform 2]: Cytoplasm. Cell projection, axon.
Protein Families
Class-II aminoacyl-tRNA synthetase family
Tissue Specificity
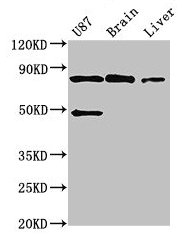
Widely expressed, including in brain and spinal cord.; [Isoform 2]: Expressed in brain, spinal cord, muscle, heart and spleen.; [Isoform 1]: Expressed in brain, spinal cord, muscle, heart, spleen and liver.