Procalcitonin is a 116-amino acid peptide precursor of the hormone calcitonin, normally produced by thyroid C-cells. Under physiological conditions, PCT levels in healthy individuals remain low. During bacterial infections and sepsis, PCT is released systemically by extrathyroidal tissues in response to bacterial endotoxins and inflammatory cytokines. This makes PCT a biomarker for distinguishing bacterial infections from viral infections and monitoring sepsis severity. PCT levels correlate with the extent of systemic inflammation and can guide antibiotic therapy decisions in clinical settings.
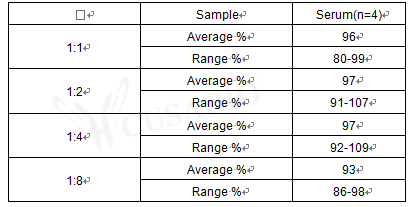
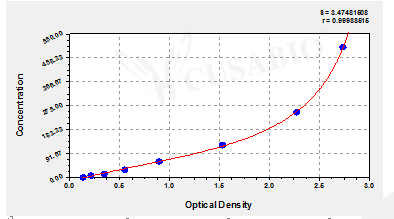
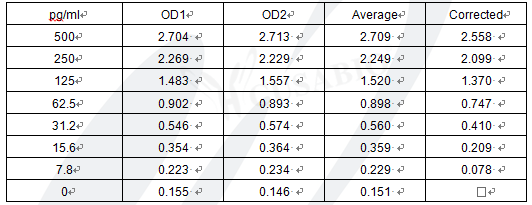
The Human Procalcitonin,PCT ELISA Kit (CSB-E09502h) is designed for quantitative measurement of PCT in human serum, plasma, and tissue homogenates. This sandwich ELISA provides a detection range of 7.8 pg/mL to 500 pg/mL with a sensitivity of 1.95 pg/mL. The assay requires 50-100 μL sample volume and can be completed within 1-5 hours, with detection performed at 450 nm wavelength.
Application Examples
Note: The following application examples are drawn from a selection of publications citing this product. For additional applications, please refer to the full list of references in the "Citations" section.
This ELISA kit has been used in clinical research studies to measure procalcitonin levels in human serum samples as part of biomarker profiling studies. The applications span infection monitoring studies and inflammatory response research where procalcitonin serves as a key analytical target alongside other clinical parameters.
• Infection monitoring: Used to track procalcitonin concentrations during different phases of infection onset and progression in clinical patient populations
• Biomarker profiling: Applied in comparative studies measuring multiple inflammatory markers including procalcitonin to evaluate disease states and clinical conditions
• Clinical parameter evaluation: Used alongside other diagnostic biomarkers to analyze patient samples and correlate procalcitonin levels with various clinical presentations