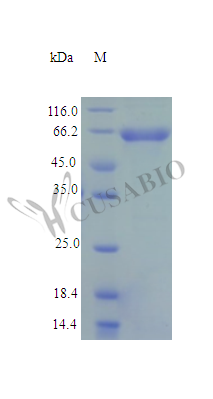
Recombinant Human NAD-dependent malic enzyme, mitochondrial protein (ME2) is produced in an E. coli expression system, covering the full-length mature protein from amino acids 19 to 584. The protein carries a C-terminal 6xHis-tag, which simplifies purification and detection processes. SDS-PAGE analysis confirms the protein achieves high purity, exceeding 95%. Biological activity remains intact, as demonstrated by spectrophotometric assay results showing a Km value of 1.5 ± 0.6 mM. Endotoxin levels stay below 1.0 EU/μg, making it appropriate for research work.
The NAD-dependent malic enzyme (ME2) appears to play a central role in mitochondrial function by converting malate to pyruvate—a step that may be critical for cellular metabolism. This reaction seems essential for maintaining NAD/NADH balance within mitochondria, which likely influences metabolic pathways, including the citric acid cycle. ME2's involvement in energy production and redox balance makes it a compelling target for research in metabolic regulation and related areas.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Enzyme Kinetics and Inhibitor Screening Studies
This application is highly valid and strongly supported. The recombinant ME2 protein has confirmed enzymatic activity with a defined Km value (1.5 ± 0.6 mM) and a standardized spectrophotometric assay. This makes it excellent for detailed kinetic studies, including substrate specificity, cofactor requirements, and inhibitor screening. The high purity (>95%) ensures reliable and reproducible results for determining kinetic parameters like Vmax, kcat, and Ki values for potential inhibitors.
2. Metabolic Pathway Analysis in Cell-Free Systems
This application is feasible with an important caveat. The active recombinant ME2 can indeed be used in reconstituted metabolic systems to study the malate-aspartate shuttle or pyruvate metabolism. However, it should be noted that while the protein is the mature human mitochondrial enzyme, it was expressed in E. coli and lacks mitochondrial localization. Therefore, while valuable for pathway biochemistry, results may not fully replicate the mitochondrial environment without additional factors.
3. Antibody Development and Validation
This application is well-supported. The high purity, low endotoxin levels, and confirmed native activity make this recombinant ME2 protein an excellent immunogen for generating specific antibodies. The C-terminal 6xHis tag facilitates purification and screening. Since the recombinant ME2 protein is functionally active, antibodies generated are more likely to recognize the native, properly folded ME2 in biological samples.
4. Protein-Protein Interaction Studies
This application is potentially feasible but requires caution. While the His-tag enables pull-down experiments, ME2 primarily functions as a metabolic enzyme rather than a scaffold protein with multiple interaction partners. The C-terminal tag position is less likely to interfere with the active site, but the relevance of identified interactions should be validated biologically. The high purity reduces background noise in such experiments.
5. Structural and Biophysical Characterization
This application is highly appropriate. The confirmed enzymatic activity indicates proper folding, making this recombinant ME2 protein suitable for biophysical studies. Techniques like analytical ultracentrifugation can investigate its oligomeric state, while thermal shift assays can study stability. The protein can also be used for structural studies using X-ray crystallography or cryo-EM, particularly to understand substrate/cofactor binding.
Final Recommendation & Action Plan
This recombinant ME2 is a high-quality, functionally validated reagent suitable for most proposed applications. The immediate priority should be leveraging its confirmed enzymatic activity for detailed kinetic studies (Application 1) and metabolic pathway reconstitution (Application 2). For interaction studies (Application 4), focus on known metabolic complexes rather than exploratory screening. The protein is excellent for antibody development (Application 3) and structural studies (Application 5). Researchers should note that while bacterial expression provides ample protein, post-translational modifications may differ from native mitochondrial ME2. All kinetic studies should use the established assay conditions as a starting point, and any identified interactors or inhibitors should be validated in cellular models.