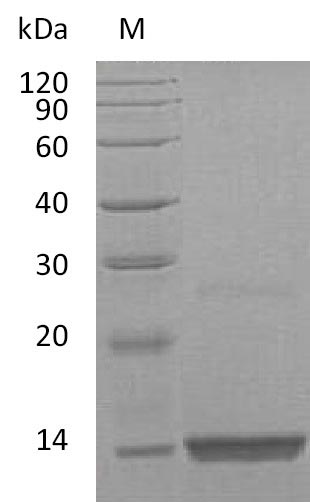
Recombinant Human Fibroblast Growth Factor 9 (FGF9) is produced in E. coli and represents the full-length protein, comprising amino acids 1-208. This tag-free preparation achieves a purity level exceeding 95% as confirmed by SDS-PAGE analysis. The protein demonstrates biological activity, with an effective dose (ED50) ranging from 1 to 5 ng/ml in a cell proliferation assay using Balb/3T3 mouse embryonic fibroblast cells. Endotoxin levels remain below 1.0 EU/µg, as determined by the LAL method.
Fibroblast Growth Factor 9 (FGF9) stands out as an important member of the fibroblast growth factor family. This protein appears to play a significant role in cellular processes such as proliferation and differentiation. FGF9 seems to be involved in various developmental pathways and may be essential for embryonic development, tissue repair, and angiogenesis. Given its apparent importance in these processes, FGF9 has become a key protein of interest in research focused on developmental biology and regenerative medicine.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Growth Factor Signaling Studies
This recombinant FGF9 is confirmed to be biologically active (ED₅₀ 1-5 ng/ml) and suitable for proliferation and signaling studies. However, researchers should validate its activity in FGF9-responsive human cell systems to complement the mouse Balb/3T3 data, as species-specific receptor affinity differences may exist. The high purity supports reliable dose-response studies, but optimal concentrations may need adjustment for primary human fibroblasts or other target cells.
2. FGF Receptor Binding and Interaction Studies
The biologically active FGF9 is appropriate for FGFR binding studies, but researchers must include heparin in binding assays as FGF9 requires heparan sulfate proteoglycans for efficient receptor dimerization and signaling. The tag-free design ensures authentic interactions, but binding kinetics should be validated in physiological conditions with heparin supplementation. The E. coli expression produces a non-glycosylated protein, but native FGF9 has minimal glycosylation, minimizing concerns.
3. Antibody Development and Validation
This high-purity, full-length FGF9 serves as an excellent antigen for antibody development. The confirmed bioactivity ensures proper folding of conformational epitopes. However, antibodies should be validated against native human FGF9 from biological sources to ensure recognition of physiological forms. The tag-free design avoids the generation of tag-specific antibodies.
4. Comparative Species and Isoform Studies
The protein enables valid comparative studies, but the activity data from mouse cells may not directly reflect potency in human systems. Comparative studies with other FGF family members should account for differences in receptor specificity (FGF9 primarily signals through FGFR3c and FGFR2c). Parallel assays in the same cell system are essential for accurate potency comparisons.
5. Protein Crystallization and Structural Biology Research
The high-purity, tag-free FGF9 is suitable for structural studies, but researchers should note that the non-glycosylated state may affect crystal packing compared to native FGF9. The confirmed bioactivity indicates proper folding, making it valuable for structural studies of the FGF9-FGFR complex, particularly when co-crystallized with heparin and receptor domains.
Final Recommendation & Action Plan
This recombinant human FGF9 is a high-quality reagent with confirmed bioactivity, making it suitable for all proposed applications. For immediate use, employ it in the 1-10 ng/ml range based on the ED₅₀, but establish dose-response curves in your specific cell systems, particularly human primary cells relevant to FGF9 biology (e.g., chondrocytes, neural cells). For binding studies, always include heparin (1-10 μg/ml) to enable proper receptor dimerization. When developing antibodies, this full-length protein is ideal for comprehensive epitope coverage, but validated against native FGF9 from human tissues. For structural studies, the protein is well-suited for crystallization trials, particularly in complex with FGFR3c and heparin. The E. coli expression producesa non-glycosylated protein, which is acceptable as FGF9 has only one potential N-glycosylation site that is not critical for function. Always include proper controls for heparin-dependent signaling and consider that different FGFR isoforms may exhibit varying affinity for FGF9. For in vivo applications, the human sequence may elicit immune responses in animal models, so consider species-matched FGF9 for long-term studies.