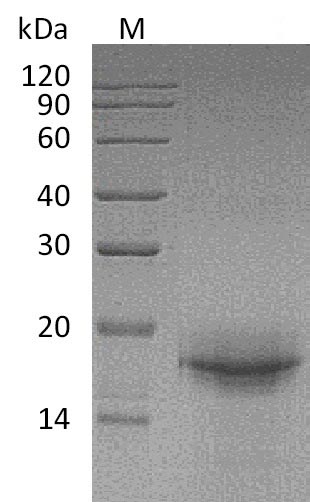
Recombinant Mouse Interleukin-33 (Il33) is produced in an E.coli system, representing a partial protein sequence from amino acids 109 to 266. This tag-free protein exhibits a high purity level of over 95%, as confirmed by SDS-PAGE analysis. It demonstrates biological activity, verified through functional ELISA, showing specific binding to Mouse ST2-Fc with an ED50 of 0.33 µg/ml. The endotoxin level is controlled, measuring less than 1.0 EU/µg according to the LAL method.
Interleukin-33 (IL-33) is a cytokine involved in immune system signaling. It acts as an alarmin—basically a molecular distress signal released when cells are damaged. This protein appears to play a critical role in inflammatory and immune responses. IL-33 binds to the receptor ST2, which then activates pathways that influence how immune cells function. Its significance in research is underscored by its involvement in various immune-related processes, potentially offering insights into inflammation mechanisms and therapeutic targets.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. IL-33/ST2 Receptor Binding Studies
This recombinant mouse IL-33 is confirmed to be biologically active in ST2 receptor binding (ED₅₀ 0.33 μg/ml for ST2-Fc) and suitable for binding studies. However, the partial sequence (109-266aa) lacks the N-terminal chromatin-binding domain of full-length IL-33, which may affect some functional aspects. The binding affinity should be validated against full-length IL-33 to ensure the truncated form maintains native-like receptor interaction characteristics. The E. coli expression produces a non-glycosylated protein, but IL-33 is not heavily glycosylated, minimizing concerns.
2. Functional ELISA Development and Optimization
The protein is appropriate as an ELISA standard, but the partial sequence may not be recognized by antibodies targeting the missing N-terminal epitopes. Researchers should validate that detection sensitivity matches full-length IL-33, particularly for complex biological samples where proteolytic processing may generate different IL-33 forms. The confirmed receptor binding indicates proper folding of the C-terminal receptor-binding domain.
3. Anti-IL-33 Antibody Development and Validation
This partial IL-33 serves as a good antigen for antibodies targeting the receptor-binding domain, but antibodies will not recognize epitopes in the missing N-terminal region (aa 1-108). Comprehensive antibody validation should include testing against full-length IL-33 to ensure detection of all biologically relevant forms. The confirmed receptor binding supports the development of function-blocking antibodies.
4. Cell-Based Functional Assays
The protein can be used for cell-based assays, but the partial sequence lacks the nuclear localization signal and chromatin-binding domain present in full-length IL-33, which are crucial for its alarmin function and nuclear signaling. Researchers should validate that cellular responses (e.g., cytokine production, signaling activation) match those induced by full-length IL-33, particularly for assays involving nuclear functions or damage-associated molecular patterns.
5. Competitive Binding and Inhibition Studies
The biologically active IL-33 is suitable for competitive binding assays, but the partial nature may affect results if inhibitors target the missing N-terminal domain. Competition studies should include full-length IL-33 controls to ensure identified inhibitors are relevant to the complete protein. The moderate binding affinity (ED₅₀ 0.33 μg/ml) suggests appropriate concentration ranges for competition experiments.
Final Recommendation & Action Plan
This recombinant mouse IL-33 partial protein (109-266aa) is a validated tool for ST2 receptor-focused studies, but its truncated nature requires specific validation steps. The protein is ideal for binding studies targeting the receptor-binding domain, but researchers should confirm that binding kinetics match full-length IL-33 for precise quantitative work. For ELISA development, this protein serves as a good standard for detecting the receptor-binding region, but should be complemented with full-length IL-33 when developing assays for comprehensive IL-33 detection. When developing antibodies, use this protein to generate reagents against the receptor-binding domain, but validate against full-length IL-33 to ensure complete epitope coverage. For cell-based assays, this protein is suitable for studying ST2-mediated signaling, but is not appropriate for studying nuclear functions or chromatin-related roles of IL-33. For inhibitor screening, the protein can identify compounds targeting the receptor interface, but hits should be validated against full-length IL-33. The E. coli expression produces non-glycosylated protein, which is acceptable as IL-33 has minimal glycosylation. Always include appropriate controls and consider that different biological contexts may require full-length IL-33 for complete functional studies.