Colony-stimulating factor 1 (CSF1), also known as macrophage colony-stimulating factor (M-CSF), is a key cytokine that plays a central role in the proliferation, differentiation, survival, and functional regulation of monocyte macrophages. In recent years, with the in-depth study of CSF1 and its receptor CSF1R (CSF1 Receptor), its role in a variety of physiological and pathological processes has gradually become clear, providing new targets and ideas for the diagnosis and treatment of related diseases. This article will review the background, mechanism of action, signaling pathways, related diseases and the progress of targeted drugs of CSF1.
1. What is CSF1
CSF1 is a secreted glycoprotein that exerts biological effects by binding to CSF1R (also known as CD115) on the cell surface. The CSF1/CSF1R signaling axis is essential in physiological processes such as the immune system, bone development, and tissue repair. Studies have shown that abnormal expression of CSF1 is closely related to the occurrence and development of a variety of diseases. Under normal physiological conditions, CSF1 is mainly secreted by fibroblasts, endothelial cells, tumor cells, etc., and regulates the development and function of macrophages. For example, CSF1 is involved in regulating the activation and proliferation of hepatic macrophages (Kupfoff cells) and promoting tissue repair during liver injury repair [1]. In the skeletal system, CSF1 is involved in the maintenance of bone metabolic balance by regulating the differentiation of osteoclasts.
2. Mechanism of action of CSF1
2.1 Regulation of cell proliferation and differentiation
After CSF1 binds to CSF1R, it promotes the differentiation of monocytes into macrophages and maintains the survival of macrophages by activating downstream signaling pathways. In tenosynovial giant cell tumor (TGCT), tumor cells overexpress CSF1 due to chromosomal translocations (eg, COL6A3-CSF1 fusion), attracting large numbers of CSF1R-positive macrophages to aggregate and form a major component of the tumor microenvironment [2,3]. This "paracrine" effect makes macrophages account for more than 90% of TGCT, while only a few are true tumor cells.
2.2 Tumor microenvironment shaping
In a variety of solid tumors, CSF1 secreted by tumor cells promotes tumor angiogenesis, immunosuppression, and metastasis by recruiting and polarizing macrophages to type M2 (pro-tumor phenotype). For example, in hepatocellular carcinoma, IL-1β-induced SLC7A11 overexpression can upregulate CSF1 expression through the HIF1α pathway, thereby promoting the infiltration and M2 polarization of tumor-associated macrophages (TAMs) and accelerating hepatocellular carcinoma metastasis [4]. In addition, in diffuse large B-cell lymphoma (DLBCL), the CREBBP/EP300 mutation promotes the polarization of TAMs to M2 through the FBXW7-NOTCH-CCL2/CSF1 axis, forming an immunosuppressive microenvironment and promoting tumor progression [5].
2.3 Immunomodulatory effects
CSF1 is involved in the regulation of innate and adaptive immune responses. In patients with asthma, CSF1 levels secreted by airway epithelial cells are significantly elevated, especially in eosinophilic asthma. CSF1 promotes airway eosinophilic inflammation and Th2-type immune response through the CSF1R-STAT1 signaling pathway, aggravating asthma symptoms [6]. In addition, in coronary heart disease, blood CSF1 levels are positively correlated with disease risk, and genetic epidemiological studies have shown that CSF1 is a causal mediator of coronary heart disease, and its mechanism may be related to CSF1-induced macrophage activation and atherosclerotic plaque formation [7].
3. CSF1-related signaling pathways
After CSF1 binds to CSF1R, it activates receptor tyrosine kinase activity and recruits JAK (Janus Kinase) family kinases to phosphorylate STAT (Signal Transducer and Activator of Transcription) protein. In HCC, CSF1/CSF1R signaling upregulates PD-L1 and CSF1 autoexpression by activating STAT1, forming a positive feedback loop that promotes tumor immune escape and metastasis [4]. In macrophages, activation of STAT3 is involved in the regulation of M2-type polarization.
This pathway plays a key role in CSF1-mediated macrophage survival and metabolic regulation. CSF1 stimulation can activate PI3K, promote AKT phosphorylation, and then activate mTOR, regulate the expression of cyclin and anti-apoptotic protein, and prolong the life of macrophages. In the Malignant Pleural Effusion (MPE) model, CSF1/CSF1R enhances macrophage vascular permeability regulation and promotes effusion formation through this pathway [8].
CSF1 signaling activates the RAS-RAF-MEK-ERK cascade and is involved in macrophage migration and functional activation. In TGCT, although tumor cells do not express CSF1R on their own, secreted CSF1 promotes the secretion of matrix metalloproteinases (e.g., MMP9) by activating the MAPK pathway that infiltrates macrophages, leading to articular cartilage destruction [2,3].
3.4 Other pathways
Aberrant expression of CSF1 in DLBCL is associated with aberrant epigenetic regulation. The CREBBP/EP300 mutation leads to a decrease in the acetylation level of H3K27, inhibits FBXW7 expression, and lifts the inhibition of the NOTCH pathway, thereby upregulating CCL2 and CSF1 and forming a pro-tumor microenvironment [5]. This epigenetic-signaling pathway interaction provides a novel explanation for the pathogenesis of DLBCL.
4. CSF1-related disease
4.1 Neoplastic diseases
4.1.1 Tendinosynovial giant cell tumor (TGCT)
TGCT is a typical representative of CSF1-related diseases and is characterized by the presence of chromosomal translocations of the CSF1 gene (e.g., COL6A3-CSF1) in tumor cells, resulting in CSF1 overexpression. Clinical studies have shown that CSF1R inhibitors (eg, pexidartinib, JNJ-40346527) can significantly reduce macrophage infiltration in TGCT, shrink tumor volume, and improve patient symptoms [2,3]. Notably, tumor cells in TGCT do not express CSF1R themselves, so CSF1R inhibitors work primarily by targeting macrophages [9].
4.1.2 Hepatocellular Carcinoma (HCC)
CSF1 is highly expressed in liver cancer tissues and promotes tumor progression by recruiting TAMs. IL-1β-induced SLC7A11 overexpression has been found to promote liver cancer metastasis through the HIF1α-CSF1 axis, while blocking the CSF1/CSF1R axis can inhibit this process [4]. In addition, the co-expression of CSF1 and PD-L1 is associated with poor prognosis in patients with liver cancer, suggesting that combined blockade of CSF1R and PD-1 may be a potential therapeutic strategy.
4.1.3 Diffuse large B-cell lymphoma (DLBCL)
The CREBBP/EP300 mutation in DLBCL promotes the polarization of TAMs to M2 through the FBXW7-NOTCH-CCL2/CSF1 axis, forming an immunosuppressive microenvironment. Clinical data have shown that high expression of CSF1 in DLBCL patients is associated with poor prognosis, and targeting the CSF1/CSF1R axis may become a new therapeutic direction [5].
4.2 Non-neoplastic diseases
4.2.1 Asthma
The airway CSF1 level in patients with eosinophilic asthma was significantly elevated, and it was positively correlated with eosinophil count and FeNO (exhaled nitric oxide). Mechanistic studies have shown that IL-13 and IL-33 can induce CSF1 secretion by airway epithelial cells and promote eosinophilic inflammation through the CSF1R-STAT1 pathway [6]. Targeting CSF1/CSF1R may provide a new treatment for severe asthma.
4.2.2 Acute Liver Failure (ALF)
Serum CSF1 levels are elevated in patients with ALF and are associated with prognosis. In animal models, CSF1-Fc fusion protein has been shown to improve ALF prognosis by enhancing the phagocytosis and repair function of hepatic macrophages [1]. CSF1 may serve as a prognostic marker and potential therapeutic target for ALF.
4.2.3 Coronary Artery Disease (CAD)
Mendelian randomization studies have demonstrated that blood CSF1 is a causal mediator of CAD, and that a genetically predicted 1 standard deviation increase in CSF1 levels is associated with an 18% increased risk of CAD [7]. CSF1 may be involved in the pathogenesis of CAD by regulating the infiltration and activation of macrophages in atherosclerotic plaques.
4.3 Other diseases
The CSF1/CSF1R axis has also been associated with diseases such as rheumatoid arthritis and malignant pleural effusion. For example, in MPE, CSF1/CSF1R signaling mediates increased vascular permeability and macrophage polarization, promoting effusion formation, and the CSF1R inhibitor BLZ945 reduces MPE volume [8]. In rheumatoid arthritis, CSF1 is involved in the activation of synovial macrophages and promotes the progression of joint inflammation [10].
5. Research progress of CSF1-targeted drugs
At present, a number of CSF1-targeted drugs are in the preclinical or clinical research stage, and have made phased progress in the exploration of different diseases, including small molecule chemical drugs, monoclonal antibodies, etc. Some of them are listed in the table below:
| Drugs |
Mechanism of action |
Type of medication |
Indications under investigation (disease name) |
Institutions under research |
Highest R&D stage |
| Cimmetinib hydrochloride |
FGFRs antagonists | M-CSF inhibitor | VEGFR2 antagonists |
Small molecule chemical drugs |
Metastatic esophageal squamous cell carcinoma | esophageal squamous cell carcinoma, etc |
Shanghai Institute of Materia Medica, Chinese Academy of Sciences | Shanghai Runshi Pharmaceutical Technology Co., Ltd. | CSPC Pharmaceutical Group Co., Ltd |
Phase 3 clinical trial |
| AUP1602-C |
M-CSF agonist | bFGF modulator |
Engineered bacteria |
Diabetic foot ulcer | Diabetic foot | Varicose vein ulcer | bedsore |
Shenzhen Unknown Jun Biotechnology Co., Ltd. | Aurealis Oy | Aurealis Therapeutics AG |
Phase 2 clinical trial |
| Lacnotuzumab |
M-CSF inhibitors |
Monoclonal antibodies |
Locally advanced melanoma |
Novartis AG |
Phase 1/2 clinical trial |
| DCR-0064 |
M-CSF inhibitors |
Small molecule chemical drugs |
tumor |
Biotechnology Development Center |
Phase 1 clinical trial |
| Anti-CD115 humanized mAb(Tasly Pharmaceutical Group) |
M-CSF inhibitors |
Monoclonal antibodies |
tumor |
Tasly Pharmaceutical Group Co., Ltd |
Preclinical |
6. CSF1 related product recommendation
The CSF1/CSF1R axis plays an important role in both physiological and pathological conditions, and its abnormalities are closely related to the occurrence and development of a variety of diseases. From TGCT to liver cancer, asthma, etc., the mechanism of action of CSF1 has been gradually elucidated, providing new targets for disease diagnosis and treatment.
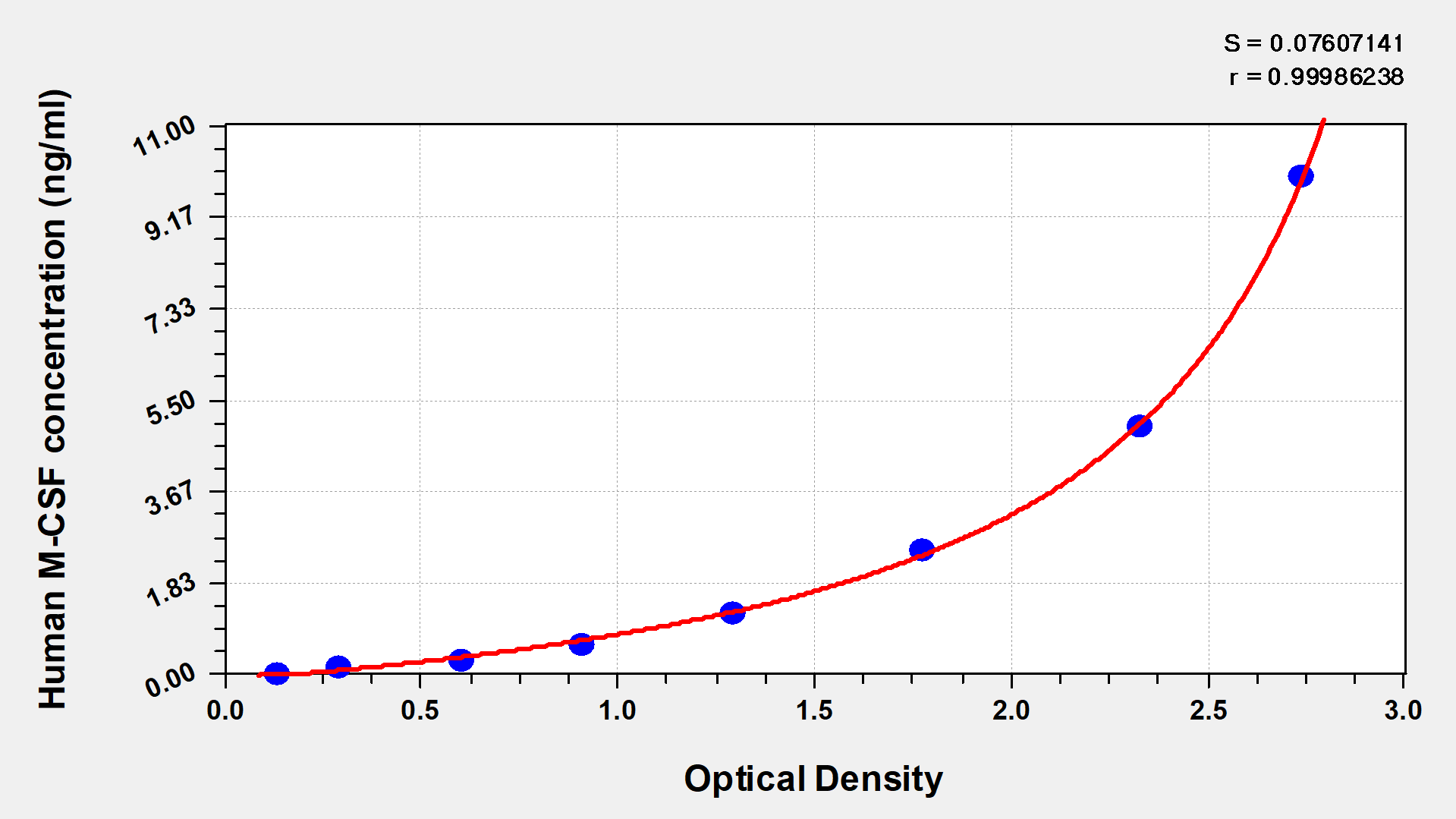
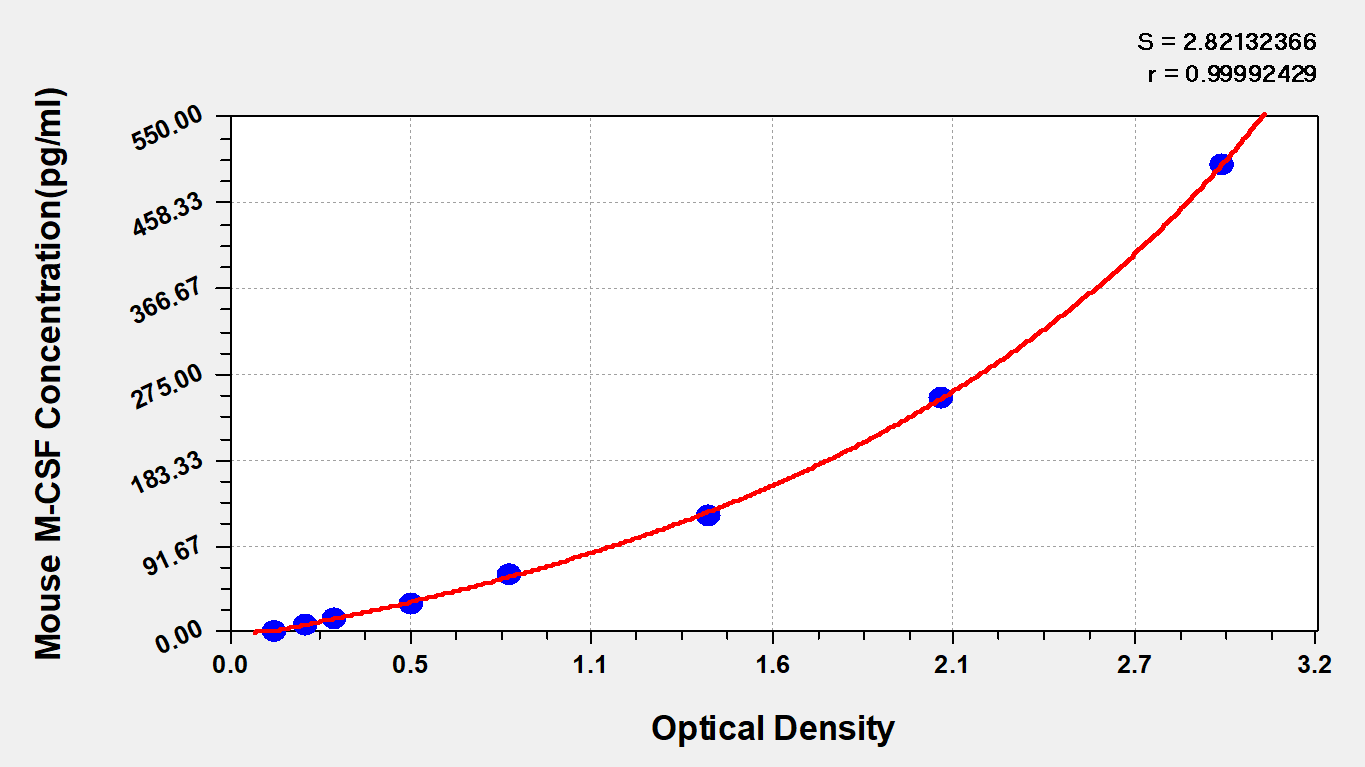
CUSABIO provides CSF1 research-related recombinant proteins, antibodies, and ELISA kit products to help your research.
► Please click here to view all CSF1 related products
References
[1] Tacke F, Wynn TA. Biomarker and Therapeutic Potential of CSF1 in Acute Liver Failure. Gastroenterology. 2015.
[2] Serrano C, et al. Safety and efficacy with vimseltinib in patients with tenosynovial giant cell tumor (TGCT) who received no prior anti–colony-stimulating factor 1 (CSF1) therapy: Ongoing phase II study. Annals of Oncology. 2024.
[3] Brahmi M, et al. Complete response to CSF1R inhibitor in a translocation variant of teno-synovial giant cell tumor without genomic alteration of the CSF1 gene. Annals of Oncology. 2018.
[4] Xia L. IL-1b-INDUCED SLC7A11 OVEREXPRESSION FACILITATES HEPATOCELLULAR CARCINOMA METASTASIS THROUGH UPREGULATING PD-L1 AND CSF1 EXPRESSION. AASLD Abstracts.
[5] Huang YH, et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor associated macrophage polarization via FBXW7-NOTCH CCL2CSF1 axis. Signal Transduction and Targeted Therapy. 2021.
[6] Du L, et al. Increased expression of CSF1 in patients with eosinophilic asthma. Immun Inflamm Dis. 2023.
[7] Sjaarda J, et al. Blood CSF1 and CXCL12 as Causal Mediators of Coronary Artery Disease. J Am Coll Cardiol. 2018.
[8] Kosti CN, et al. CSF1/CSF1R signaling mediates malignant pleural effusion formation. JCI Insight. 2022.
[9] van IJzendoorn DGP, et al. Interactions in CSF1-Driven Tenosynovial Giant Cell Tumors. Clin Cancer Res. 2022.
[10] Saleh R, et al. CSF-1 in inflammatory and arthritic pain development. J Immunol. 2018.
CUSABIO team. Colony Stimulating Factor 1 (CSF1): Its Key Role in Diseases and New Progress in Targeted Therapy. https://www.cusabio.com/c-21239.html


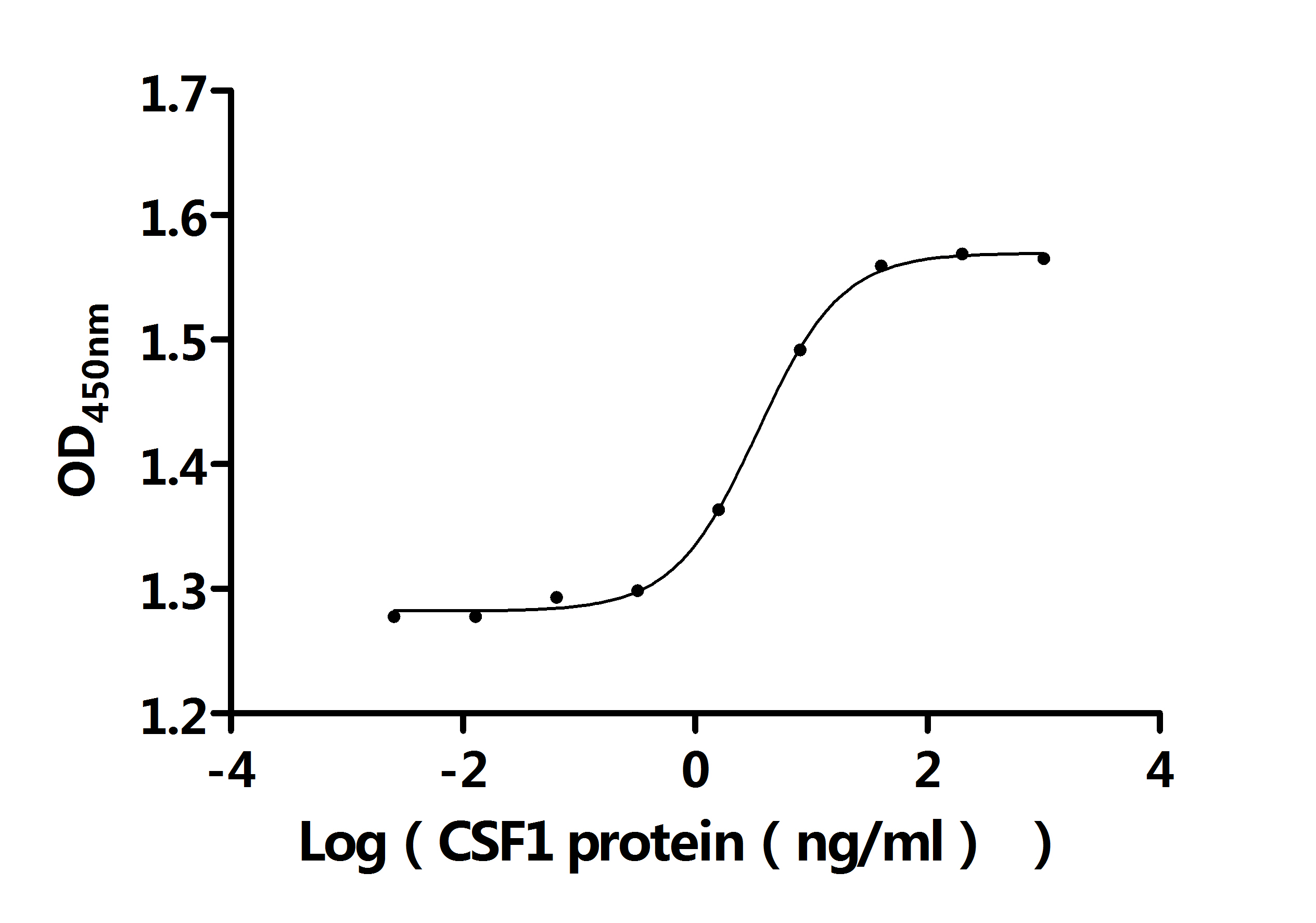
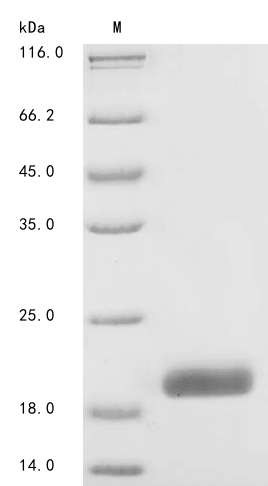
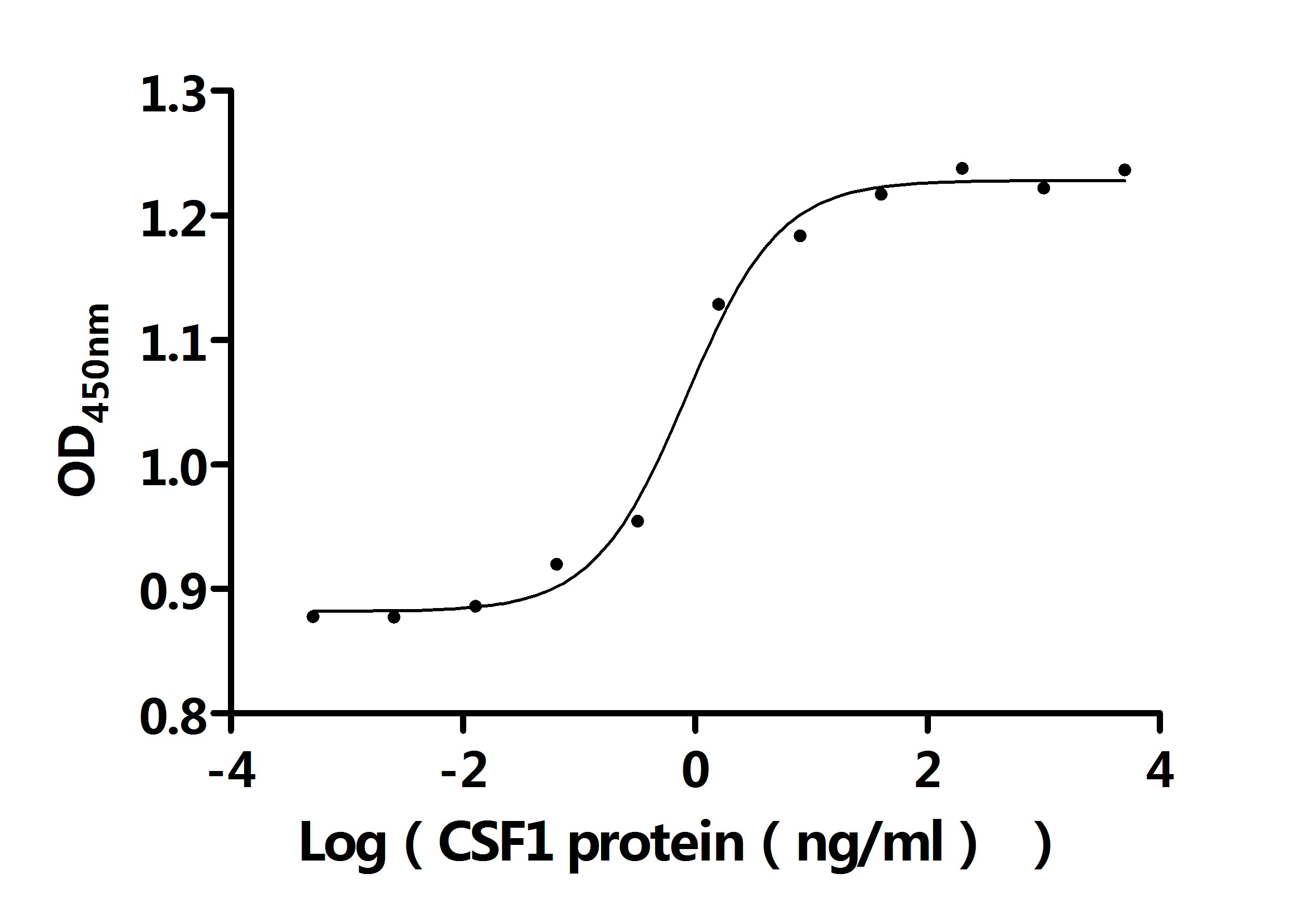



Comments
Leave a Comment