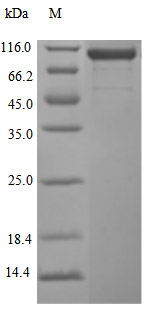
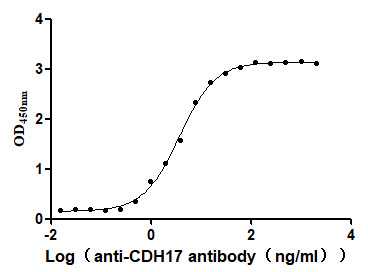
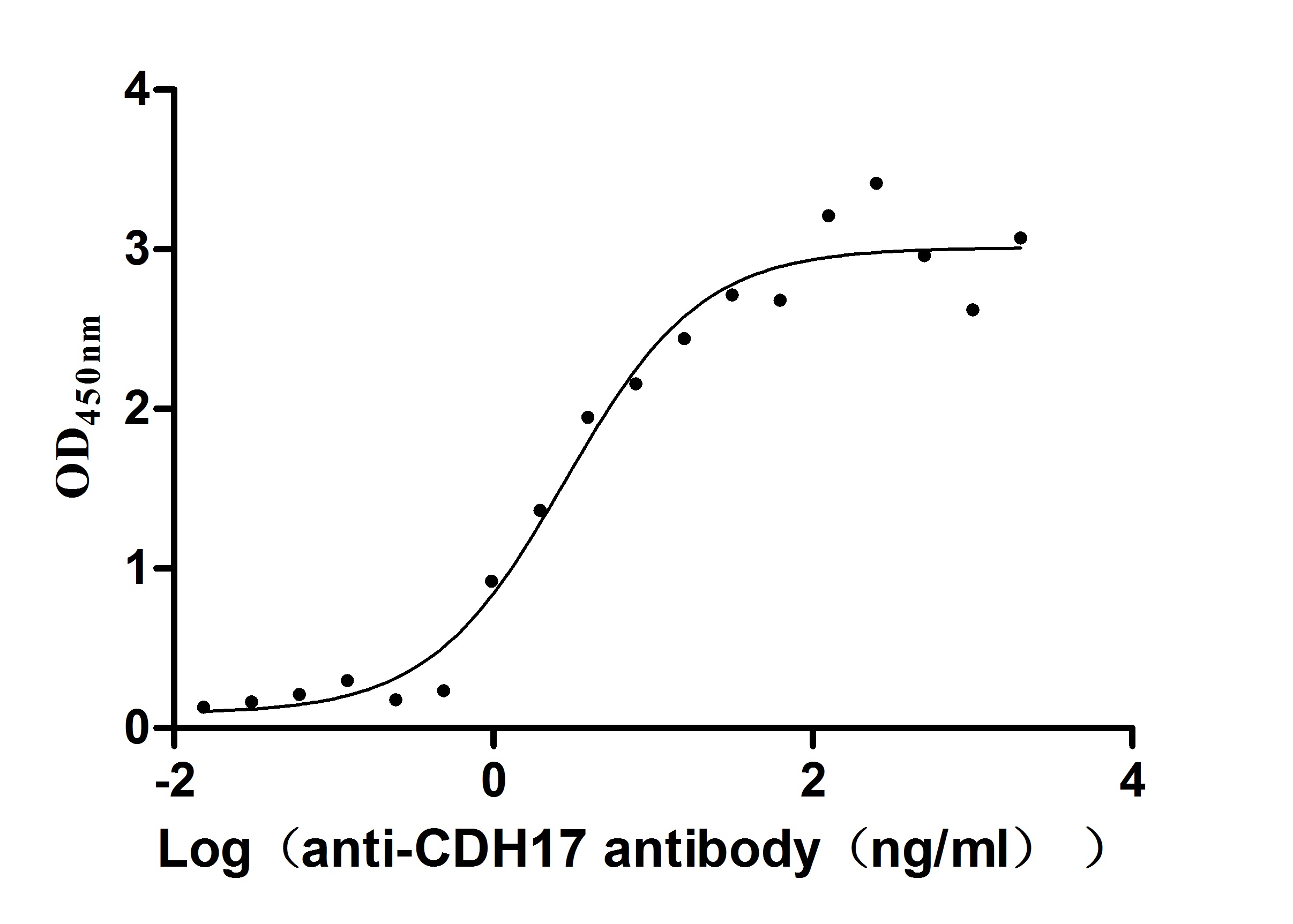
Recombinant human CDH17 protein production includes cloning the target gene that encodes the 23-787aa of the human CDH17, constructing a plasmid that contains the target gene, expressing the protein in mammalian cells, and conducting purification. It is fused with a C-terminal 10xHis-tag. The recombinant CDH17 protein is purified via Ni-NTA affinity chromatography. Its purity is over 96% as determined by SDS-PAGE, and its endotoxin content is measured at <1.0 EU/μg by the LAL assay. Functional ELISA demonstrates its binding with the CDH17 recombinant antibody (CSB-RA613267MA1HU), with an EC50 of 3.095 to 4.451 ng/mL.
Human CDH17, also known as liver-intestine cadherin (LI-cadherin), is a member of the cadherin superfamily, which comprises a group of proteins that play crucial roles in cell adhesion and signaling. Structurally, CDH17 is characterized by seven extracellular cadherin domains and a notably short cytoplasmic tail of only 20 amino acids, distinguishing it from classical cadherins that typically possess five extracellular domains and a longer cytoplasmic tail [1][2]. This unique structure allows CDH17 to function as a calcium-dependent homophilic cell adhesion molecule, primarily expressed on the basolateral surface of enterocytes and hepatocytes in humans [2][3].
The expression of CDH17 is predominantly observed in the fetal liver and gastrointestinal tract, but it is downregulated in adult tissues. However, its expression is significantly upregulated in various malignancies, including gastric cancer, colorectal cancer, and hepatocellular carcinoma [4][5][6]. In gastric cancer, CDH17 expression correlates with tumor progression and poor prognosis, as it is associated with deeper tumor invasion and lymph node metastasis [6][7]. Studies have shown that CDH17 knockdown in colorectal cancer leads to reduced proliferation and colony-forming ability [8].
CDH17 is also implicated in various signaling pathways that influence tumor growth and metastasis. CDH17 modulates beta-catenin signaling, which is critical in the Wnt signaling pathway, a pathway often dysregulated in cancer [9][10]. Furthermore, CDH17's involvement in immune responses has been explored, with studies suggesting its potential as a target for immunotherapy in colorectal cancer [8][11].
References:
[1] J. Ma, X. Xu, C. Fu, P. Xia, M. Tian, L. Zheng, et al. Cdh17 nanobodies facilitate rapid imaging of gastric cancer and efficient delivery of immunotoxin, Biomaterials Research, vol. 26, no. 1, 2022. https://doi.org/10.1186/s40824-022-00312-3
[2] H. Lee, K. Nam, H. Park, M. Kim, B. LaFleur, H. Aburatani, et al. Gene expression profiling of metaplastic lineages identifies cdh17 as a prognostic marker in early stage gastric cancer, Gastroenterology, vol. 139, no. 1, p. 213-225.e3, 2010. https://doi.org/10.1053/j.gastro.2010.04.008
[3] Z. Bao, S. Shen, K. Wong, G. Zi-tong, W. Sun, X. Ni, et al. Clinical correlation of cadherin‐17 marker with advanced tumor stages and poor prognosis of cholangiocarcinoma, Journal of Surgical Oncology, vol. 123, no. 5, p. 1253-1262, 2021. https://doi.org/10.1002/jso.26399
[4] S. Funakoshi, T. Shimizu, O. Numata, M. Ato, F. Melchers, & K. Ohnishi. Bill-cadherin/cadherin-17 contributes to the survival of memory b cells, Plos One, vol. 10, no. 1, p. e0117566, 2015. https://doi.org/10.1371/journal.pone.0117566
[5] Y. Ding. Cadherin 17 nanobody-mediated near-infrared-ii fluorescence imaging-guided surgery and immunotoxin delivery for colorectal cancer, Biomaterials Research, vol. 28, 2024. https://doi.org/10.34133/bmr.0041
[6] R. Ito, N. Oue, K. Yoshida, K. Kunimitsu, H. Nakayama, K. Nakachi, et al. Clinicopathological significant and prognostic influence of cadherin-17 expression in gastric cancer, Virchows Archiv, vol. 447, no. 4, p. 717-722, 2005. https://doi.org/10.1007/s00428-005-0015-2
[7] H. Wang. Cadherin dysregulation in gastric cancer: insights into gene expression, pathways, and prognosis, Journal of Gastrointestinal Oncology, vol. 14, no. 05, p. 2064-2082, 2023. https://doi.org/10.21037/jgo-23-700
[8] K. Wong. Cdh17 as a cell surface immunotherapy target in colorectal cancer: an in silico analysis,, 2022. https://doi.org/10.21203/rs.3.rs-1894271/v1
[9] Y. Wang, F. Shek, K. Wong, L. Liu, X. Zhang, Y. Yuan, et al. Anti-cadherin-17 antibody modulates beta-catenin signaling and tumorigenicity of hepatocellular carcinoma, Plos One, vol. 8, no. 9, p. e72386, 2013. https://doi.org/10.1371/journal.pone.0072386
[10] L. Liu, N. Lee, V. Chan, W. Xue, L. Zender, C. Zhang, et al. Targeting cadherin-17 inactivates wnt signaling and inhibits tumor growth in liver carcinoma, Hepatology, vol. 50, no. 5, p. 1453-1463, 2009. https://doi.org/10.1002/hep.23143
[11] J. García‐Martínez, S. Wang, C. Weishaeupl, A. Wernitznig, P. Chetta, C. Pinto, et al. Selective tumor cell apoptosis and tumor regression in cdh17-positive colorectal cancer models using bi 905711, a novel liver-sparing trailr2 agonist, Molecular Cancer Therapeutics, vol. 20, no. 1, p. 96-108, 2021. https://doi.org/10.1158/1535-7163.mct-20-0253