[1] Hulpiau, Paco, Ismail Sahin Gul, and Frans Van Roy. "Evolution of cadherins and associated catenins. "The Cadherin Superfamily: Key Regulators of Animal Development and Physiology (2016): 13-37.
[2] Ma, Yi-Shih, et al. "Bisdemethoxycurcumin suppresses human osteosarcoma U-2 OS cell migration and invasion via affecting the PI3K/Akt/ NF-κB, PI3K/Akt/GSK3β and MAPK signaling pathways in vitro." Oncology Reports 48.6 (2022): 1-10.
[3] Yu, Weina, et al. "Cadherin signaling in cancer: its functions and role as a therapeutic target." Frontiers in oncology 9 (2019): 989.
[4] Türkeli, Ahmet, et al. "Anti-VEGF treatment suppresses remodeling factors and restores epithelial barrier function through the E -cadherin/β-catenin signaling axis in experimental asthma models." Experimental and Therapeutic Medicine 22.1 (2021): 1 -9.
[5] Ramesh, P. K. Immunolocalization of Gingival E-Cadherin Expression in Smokers and Non-Smokers with Chronic Periodontitis. diss. KSR Institute of Dental Science and Research, Tiruchengode, 2020.
[6] Harki, Olfa, et al. "Inhibition of Vascular Endothelial Cadherin Cleavage Prevents Elastic Fiber Alterations and Atherosclerosis Induced by Intermittent Hypoxia in the Mouse Aorta." International Journal of Molecular Sciences 23.13 (2022): 7012.
[7] Koziolek, Michael, et al. "Urine E-cadherin: a marker for early detection of kidney injury in diabetic patients." journal of clinical medicine 9.3 (2020 ): 639.
[8] Loh, Chin-Yap, et al. "The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges." Cells 8.10 (2019): 1118.
[9] Yu, Chong, et al. "The lncRNA ZNF667-AS1 Inhibits Propagation, Invasion, and Angiogenesis of Gastric Cancer by Silencing the Expression of N-Cadherin and VEGFA." Journal of Oncology 2022 (2022).
[10] Zhang, Jinyao, et al. "Clinical significance of ALDH1A1 expression and its association with E-cadherin and N-cadherin in resected large cell neuroendocrine carcinoma." Translational Oncology 19 (2022): 101379.
[11] Takamura, Masaaki, et al. "Involvement of liver-intestine cadherin in cancer progression." medical molecular morphology 46 (2013): 1-7.
[12] Ordóñez, Nelson G. "Cadherin 17 is a novel diagnostic marker for adenocarcinomas of the digestive system." Advances in anatomic pathology 21.2 (2014): 131-137.
[13] Gray, Michelle E., and Marcos Sotomayor. "Crystal structure of the nonclassical cadherin-17 N-terminus and implications for its adhesive binding mechanism." Acta Crystallographica Section F: Structural Biology Communications 77.3 (2021): 85-94.
[14] Caporuscio, Christian, et al. "Immunoaffinity enrichment LC-MS/MS quantitation of CDH17 in tissues." Bioanalysis 12.20 (2020): 1439- 1447.
[15] Horsfield, Julia, et al. "Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development." Mechanisms of development 115.1-2 (2002): 15-26.
[16] Huang, Li-Ping, et al. "Up-regulation of cadherin 17 and down-regulation of homeodomain protein CDX2 correlate with tumor progression and unfavorable prognosis in epithelial ovarian cancer." International Journal of Gynecologic Cancer 22.7 (2012).
[17] Jiang, Xiao-jie, et al. "CDH17 alters MMP-2 expression via canonical NF-κB signalling in human gastric cancer." Gene 682 (2019): 92-100.
[18] Liu, Xinjian, et al. "Disruption of oncogenic liver-intestine cadherin (CDH17) drives apoptotic pancreatic cancer death." Cancer Letters 454 (2019): 204-214.
[19] Xia, Peng, et al. "Surface-Engineered Extracellular Vesicles with CDH17 Nanobodies to Efficiently Deliver Imaging Probes and Chemo -Photothermal Drugs for Gastric Cancer Theragnostic." Advanced Functional Materials (2022): 2209393.
[20] Xia, Peng, et al. "Surface-Engineered Extracellular Vesicles with CDH17 Nanobodies to Efficiently Deliver Imaging Probes and Chemo -Photothermal Drugs for Gastric Cancer Theragnostic." Advanced Functional Materials (2022): 2209393.
[21] Feng, Zijie, et al. "Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues." Nature Cancer 3.5 (2022): 581-594.
[22] Pei, Xiao Meng, et al. "The diagnostic significance of CDH17-positive circulating tumor cells in patients with colorectal cancer." Expert Review of Molecular Diagnostics just-accepted (2023).
[23] Wu, Cunen, et al. "Interaction between Wnt/β-catenin pathway and microRNAs regulates epithelial-mesenchymal transition in gastric cancer." International journal of oncology 48.6 (2016): 2236-
[22] 6.
[24] Kaszak, Ilona, et al. "Role of cadherins in cancer-a review." international journal of molecular sciences 21.20 (2020): 7624.
[25] Wang, Qianwen, et al. "MICAL2 contributes to gastric cancer cell migration via Cdc42-dependent activation of E-cadherin/β-catenin signaling pathway ." Cell Communication and Signaling 20.1 (2022): 136.
[26] Lee, Nikki P., et al. "Role of cadherin-17 in oncogenesis and potential therapeutic implications in hepatocellular carcinoma. "Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1806.2 (2010): 138-145.
[27] Huang, Hsiang-Wei, et al. "Association between inflammation and function of cell adhesion molecules influence on gastrointestinal cancer development." Cells 10.1 (2021): 67.
[28] Maher, John, and David M. Davies. "CAR-Based Immunotherapy of Solid Tumours-A Survey of the Emerging Targets. "Cancers 15.4 (2023): 1171.
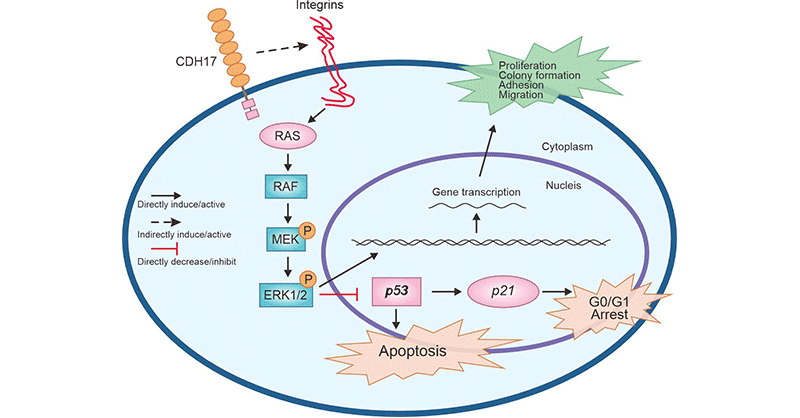
[29] Lin, Zhaohu, et al. "Targeting cadherin-17 inactivates Ras/Raf/MEK/ERK signaling and inhibits cell proliferation in gastric cancer." PLoS One 9.1 ( 2014): e85296.
[30] Wang, Yonggang, et al. "Anti-cadherin-17 antibody modulates beta-catenin signaling and tumorigenicity of hepatocellular carcinoma." ploS one 8.9 ( 2013): e72386.
[31] Motoshita, Junichi, et al. "Molecular characteristics of differentiated-type gastric carcinoma with distinct mucin phenotype: LI -cadherin is associated with intestinal phenotype." Pathology international 56.4 (2006): 200-205.
[32] Wang, Jin, et al. "Cadherin-17 induces tumorigenesis and lymphatic metastasis in gastric cancer through activation of NFκB signaling pathway." Cancer biology & therapy 14.3 (2013): 262-270.
[33] Qiu, Hai-bo, et al. "Targeting CDH17 suppresses tumor progression in gastric cancer by downregulating Wnt/β-catenin signaling." ploS one 8.3 (2013): e56959.
[34] Su, Min-Cheng, et al. "Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system." Modern Pathology 21.11 (2008): 1379- 1386.
[35] Takamura, Masaaki, et al. "Loss of liver-intestine cadherin in human intrahepatic cholangiocarcinoma promotes angiogenesis by up-regulating metal- responsive transcription factor-1 and placental growth factor." International journal of oncology 36.1 (2010): 245-254.
[36] Harding, J. J., et al. "371P A phase Ia/b, open-label, multicentre study of the TRAILR2 agonist BI 905711 in patients (pts) with advanced gastrointestinal (GI) cancers." Annals of Oncology 33 (2022): S706.
[37] Panarelli, Nicole C., et al. "Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2 ." American journal of clinical pathology 138.2 (2012): 211-222.
[38] Shenoy, U. Sangeetha, et al. "Molecular implications of HOX genes targeting multiple signaling pathways in cancer." Cell biology and toxicology (2022) : 1-30. : 1-30.
[39] Ignatova, Ekaterina Olegovna, et al. "Clinical significance of molecular subtypes of gastrointestinal tract adenocarcinoma." World Journal of Gastrointestinal Oncology 14.3 (2022): 628.
[40] Liu, Xinjian, et al. "Disruption of oncogenic liver-intestine cadherin (CDH17) drives apoptotic pancreatic cancer death." Cancer Letters 454 (2019): 204-214.
[41] Huang, Li-Ping, et al. "Up-regulation of cadherin 17 and down-regulation of homeodomain protein CDX2 correlate with tumor progression and unfavorable prognosis in epithelial ovarian cancer." International Journal of Gynecologic Cancer 22.7 (2012).
[42] Karimi, S. S., T. Valyi-Nagy, and M. F. Gonzalez. "TTF-1 Immunoexpression in Primary Rectal Adenocarcinoma with Brain Metastasis. "American Journal of Clinical Pathology 156.Supplement_1 (2021): S63-S63.

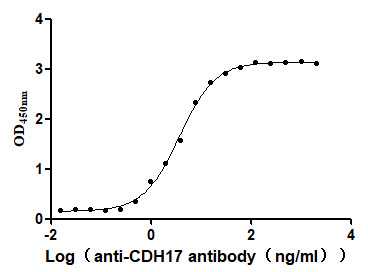
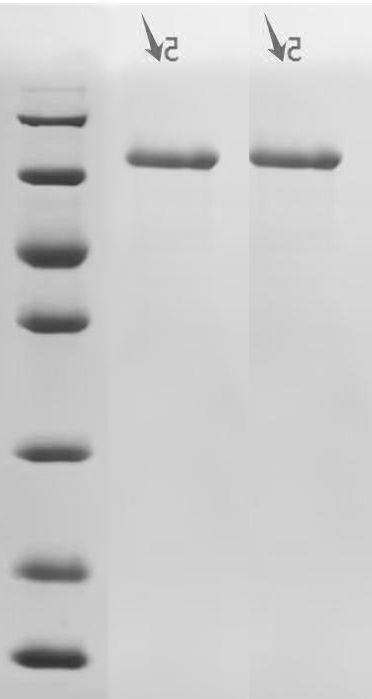
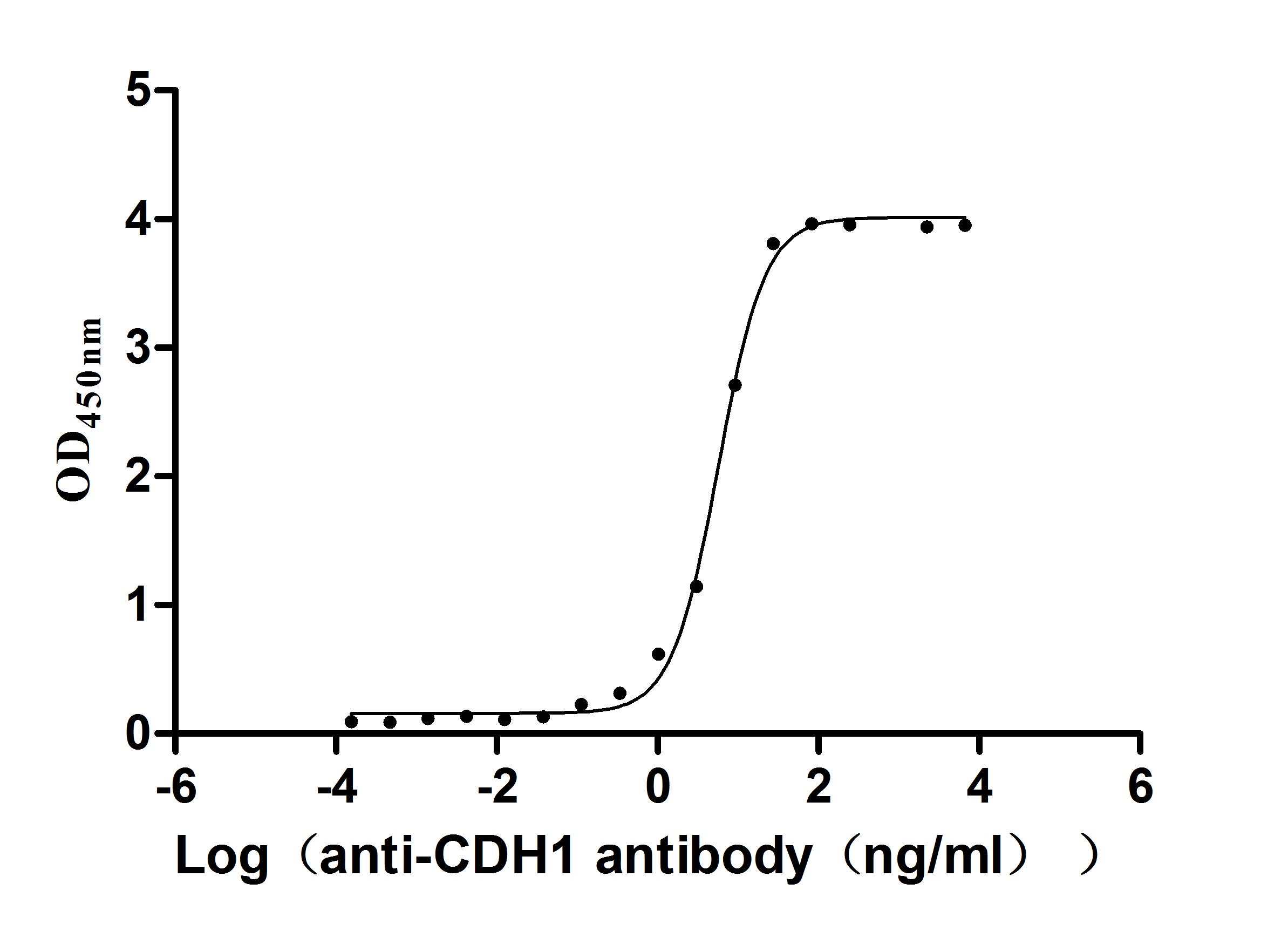

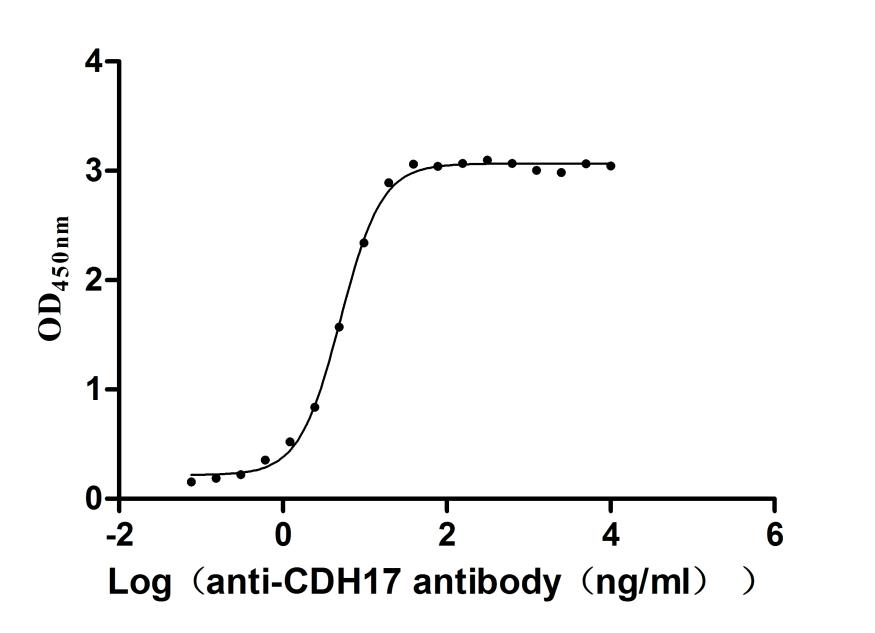
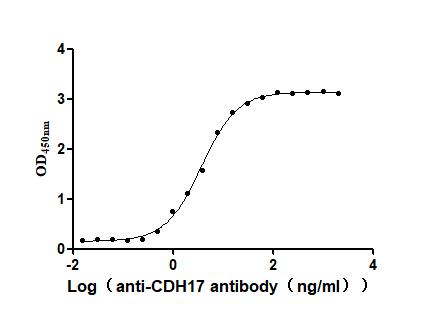
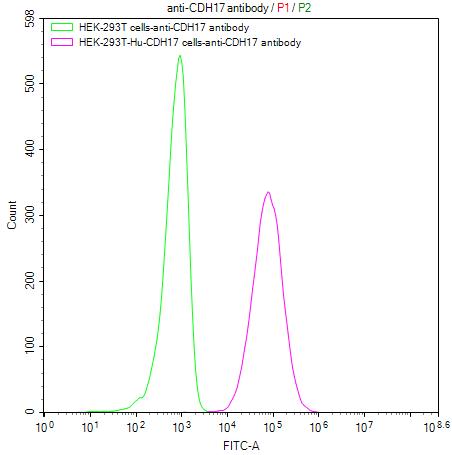
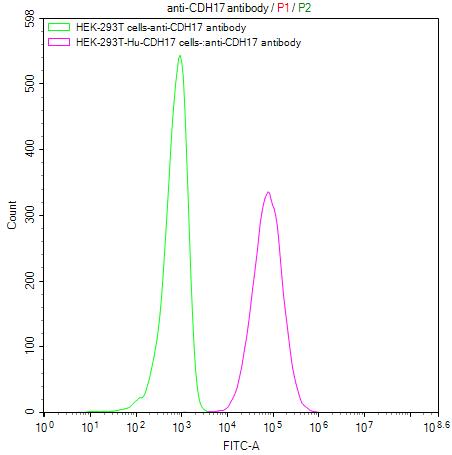
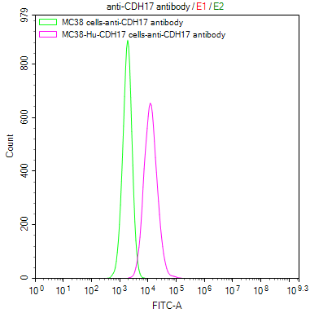


Comments
Leave a Comment