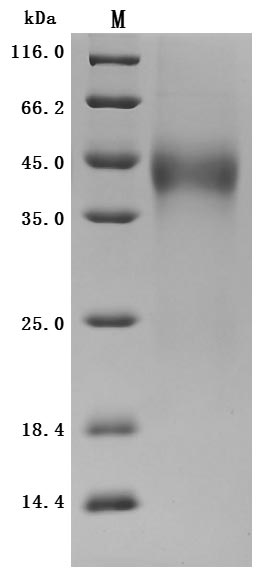
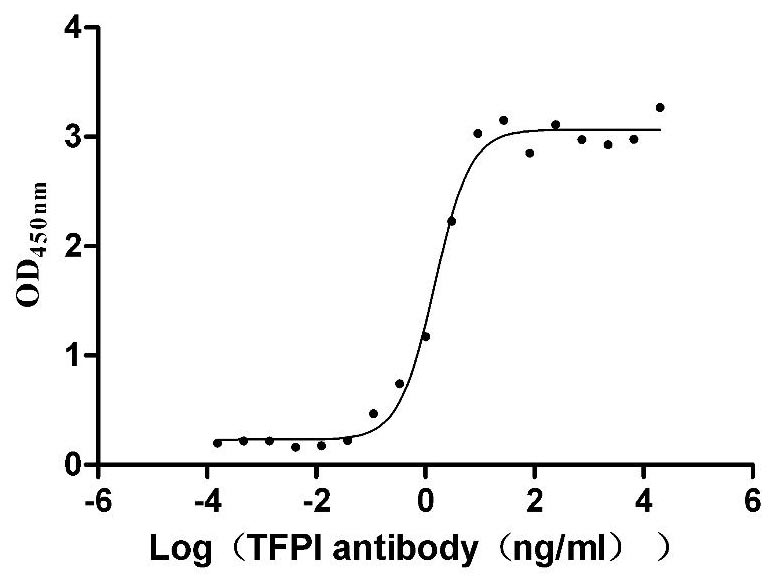
This recombinant human TFPI protein (amino acids 29-282) is produced in mammalian cells with a C-terminal 10×His tag, demonstrating high purity (>95% by SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method). The recombinant TFPI protein exhibits strong biological activity, as evidenced by its specific binding to anti-TFPI antibody (CSB-RA023437MA01HU) in ELISA (EC50: 1.242-1.788 ng/mL at 1 μg/mL immobilization).
The mammalian expression system ensures proper folding and post-translational modifications critical for TFPI's anticoagulant function. The C-terminal 10×His tag facilitates purification while maintaining structural integrity. Presented as lyophilized powder, this preparation offers excellent stability and convenient handling.
The protein's demonstrated binding activity and high purity make it suitable for functional assays exploring TFPI's role in regulating tissue factor-mediated blood coagulation. Its mammalian origin ensures native-like characteristics for reliable experimental results in both basic and translational research applications.
Human TFPI is a critical regulator of the coagulation cascade, primarily functioning to limit the procoagulant activity initiated by the tissue factor (TF) and the factor VIIa (FVIIa) complex. TF is a transmembrane protein that, upon interaction with FVIIa, activates factors IX and X, leading to thrombin generation and subsequent clot formation. TFPI acts as an anticoagulant by binding to the TF-FVIIa complex and inhibiting factor Xa (FXa), thereby preventing further propagation of the coagulation cascade [1][2][3].
TFPI is categorized as a Kunitz-type serine protease inhibitor and exists in two forms: a membrane-bound variant on endothelial cells and a soluble form found in blood plasma [1][4]. This duality allows TFPI to perform immediate regulatory functions at sites of vascular injury while maintaining anticoagulant activity in circulation. The physiological importance of TFPI arises from its ability to modulate thrombus formation and may impact conditions such as atherosclerosis or acute thrombotic events [5][6].
The structure of TFPI includes three Kunitz-type domains, functioning together to ensure that it efficiently inhibits both the FVIIa-TF complex and FXa, the latter through a feedback mechanism that effectively halts the coagulation process [3][7]. The interaction between TFPI and FXa also leads to the formation of quaternary complexes that enhance the inhibition of the coagulation pathway [8]. Notably, under certain pathological conditions such as disseminated intravascular coagulation (DIC), TFPI levels may decrease, leading to enhanced thrombin generation and a hypercoagulable state [9][10].
Furthermore, TFPI's concentration in plasma can be influenced by several clinical conditions, including critical limb ischemia and various thrombophilic states [6][11][12]. The regulatory role of TFPI in inflammation and disease states further emphasizes its significance, positioning it as a potential therapeutic target in managing thromboembolic disorders [1][10][13].
References:
[1] E. Alan. Alternatively spliced tissue factor pathway inhibitor: functional implications. Frontiers in Bioscience-Elite, vol. S3, no. 1, p. 1457, 2011. https://doi.org/10.2741/236
[2] P. Stalboerger, C. Panetta, R. Simari, & N. Caplice. Plasmin proteolysis of endothelial cell and vessel wall associated tissue factor pathway inhibitor. Thrombosis and Haemostasis, vol. 86, no. 09, p. 923-928, 2001. https://doi.org/10.1055/s-0037-1616151
[3] M. Ndonwi, T. Girard, & G. Broze. The c‐terminus of tissue factor pathway inhibitor α is required for its interaction with factors v and va. Journal of Thrombosis and Haemostasis, vol. 10, no. 9, p. 1944-1946, 2012. https://doi.org/10.1111/j.1538-7836.2012.04834.x
[4] D. Eitzman, R. Westrick, et al. Lethal perinatal thrombosis in mice resulting from the interaction of tissue factor pathway inhibitor deficiency and factor v leiden. Circulation, vol. 105, no. 18, p. 2139-2142, 2002. https://doi.org/10.1161/01.cir.0000017361.39256.82
[5] T. Koudriavtseva, S. Lorenzano, et al. Tissue factor as a potential coagulative/vascular marker in relapsing-remitting multiple sclerosis. Frontiers in Immunology, vol. 14, 2023. https://doi.org/10.3389/fimmu.2023.1226616
[6] R. Wieczór, A. Kulwas, & D. Rość. Implications of hemostasis disorders in patients with critical limb ischemia—an in-depth comparison of selected factors. Journal of Clinical Medicine, vol. 9, no. 3, p. 659, 2020. https://doi.org/10.3390/jcm9030659
[7] M. Ragni, P. Golino, et al. Endogenous tissue factor pathway inhibitor modulates thrombus formation in an in vivo model of rabbit carotid artery stenosis and endothelial injury. Circulation, vol. 102, no. 1, p. 113-117, 2000. https://doi.org/10.1161/01.cir.102.1.113
[8] A. Kumar, K. Koenig, A. Johnson, D. Fair, & S. Idell. Inhibition of factor xa-mediated procoagulant activity of human lung fibroblasts and pleural mesothelial cells. European Respiratory Journal, vol. 8, no. 12, p. 2038-2045, 1995. https://doi.org/10.1183/09031936.95.08122038
[9] T. Geisbert, L. Hensley, et al. Treatment of ebola virus infection with a recombinant inhibitor of factor viia/tissue factor: a study in rhesus monkeys. The Lancet, vol. 362, no. 9400, p. 1953-1958, 2003. https://doi.org/10.1016/s0140-6736(03)15012-x
[10] P. Enkhbaatar, K. Okajima, et al. Recombinant tissue factor pathway inhibitor reduces lipopolysaccharide-induced pulmonary vascular injury by inhibiting leukocyte activation. American Journal of Respiratory and Critical Care Medicine, vol. 162, no. 5, p. 1752-1759, 2000. https://doi.org/10.1164/ajrccm.162.5.9911018
[11] J. Dennis, V. Trương, et al. Single nucleotide polymorphisms in an intergenic chromosome 2q region associated with tissue factor pathway inhibitor plasma levels and venous thromboembolism. Journal of Thrombosis and Haemostasis, vol. 14, no. 10, p. 1960-1970, 2016. https://doi.org/10.1111/jth.13431
[12] C. Duckers, P. Simioni, et al. Low plasma levels of tissue factor pathway inhibitor in patients with congenital factor v deficiency. Blood, vol. 112, no. 9, p. 3615-3623, 2008. https://doi.org/10.1182/blood-2008-06-162453
[13] S. Stojković, C. Kaun, et al. Tissue factor is induced by interleukin-33 in human endothelial cells: a new link between coagulation and inflammation. Scientific Reports, vol. 6, no. 1, 2016. https://doi.org/10.1038/srep25171