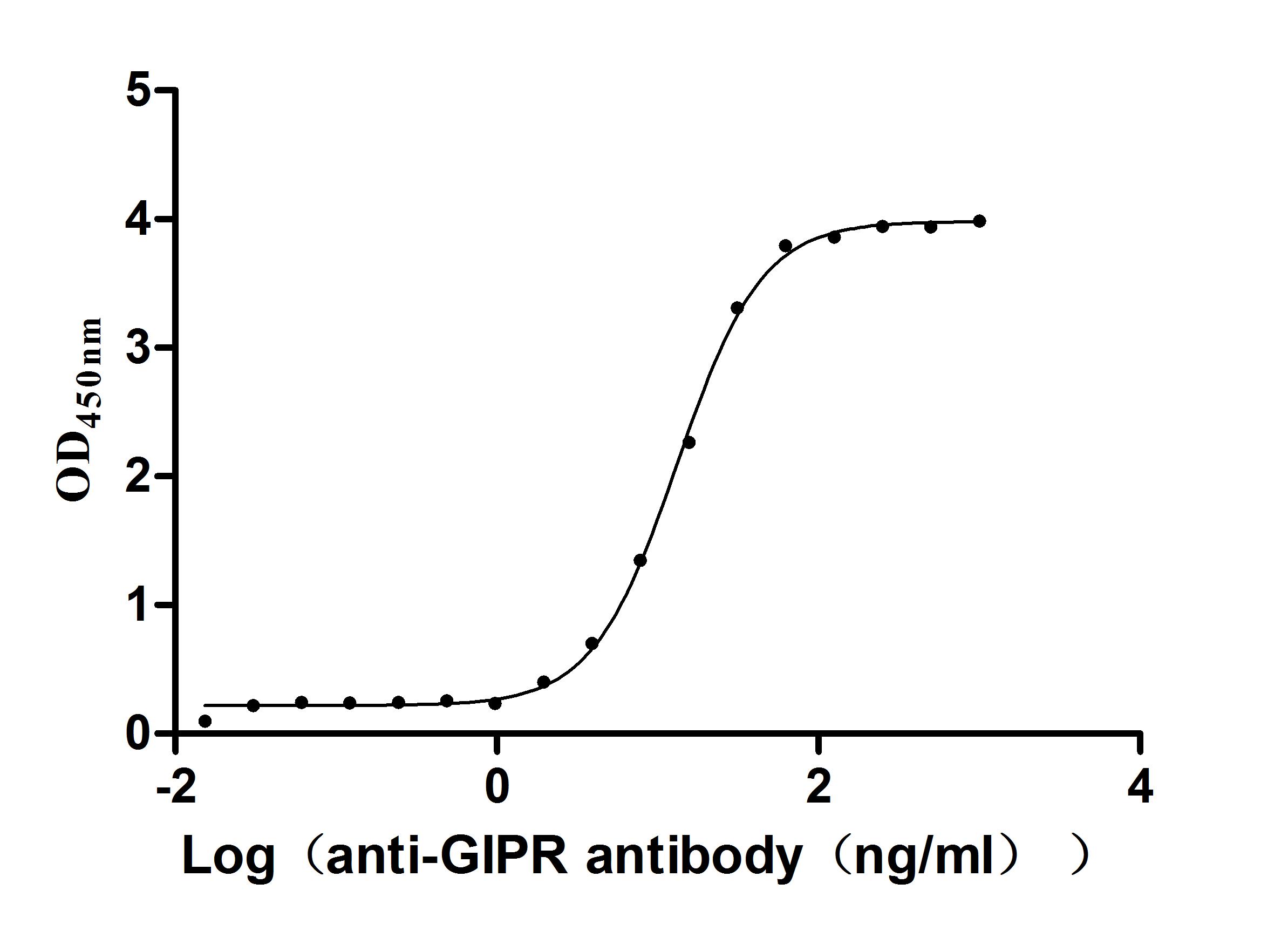
This active recombinant Macaca fascicularis gastric inhibitory polypeptide receptor (GIPR) fragment (amino acid residues 26-138) is expressed in yeast with an N-terminal 6×His tag, achieving high purity (>95% by SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method). Functional validation demonstrates specific binding to anti-GIPR recombinant antibody (CSB-RA009438MA1HU) in ELISA, with the EC50 of 12.66–13.86 ng/mL, confirming proper folding and antigenic competence. Its lyophilized form facilitates enhanced stability and is convenient for storage and transportation. The His tag ensures efficient purification without disrupting ligand-binding domains. This GIPR protein is valuable for probing GIPR's function in energy homeostasis and β-cell biology.
The GIPR protein, known for its role in glucose metabolism regulation, has been studied within various species, including Macaca fascicularis (cynomolgus macaque). GIPR functions primarily as a receptor for the endogenous hormone, gastric inhibitory peptide (GIP), which is implicated in insulin secretion and appetite regulation. This modulation is crucial as it complements the effects of incretin hormones, thus influencing glucose levels and energy homeostasis [1].
In Macaca fascicularis, the GIPR protein exhibits similarities with its counterparts in other primate species. For instance, research has demonstrated that nonhuman primates like the cynomolgus macaque provide a relevant model for studying obesity and diabetes due to their physiological responses akin to humans [2]. This genetic and physiological proximity offers valuable insights into alterations in GIPR signaling that could manifest in metabolic disorders.
Moreover, the role of GIPR in the context of immune responses has begun to gain attention. It has been suggested that beyond its metabolic functions, GIPR may also interact with various immune pathways, intersecting with the functions of different cytokines and chemokines present in the immune response [2][3]. This is particularly relevant, as immune modulation could influence the pathophysiological conditions in which GIPR might be involved, such as chronic inflammation or cancer, given the shared pathways between metabolism and immune functionality [4][1].
Most importantly, studies have indicated that variations in GIPR may contribute to the differences observed in glucose metabolism and susceptibility to diabetes among primate species. In the specific context of Macaca fascicularis, its GIPR structure and function underline its potential as a therapeutic target for managing diabetes. Research utilizing cynomolgus macaques in drug development has shown that a detailed understanding of genetic expressions and variability can lead to better-focused interventions for human diseases [5][6].
References:
[1] S. Kanthaswamy, J. Ng, et al. The genetic composition of populations of cynomolgus macaques (macaca fascicularis) used in biomedical research. Journal of Medical Primatology, vol. 42, no. 3, p. 120-131, 2013. https://doi.org/10.1111/jmp.12043
[2] J. Estes, S. Wong, & J. Brenchley,. Nonhuman primate models of human viral infections. Nature Reviews Immunology, vol. 18, no. 6, p. 390-404, 2018. https://doi.org/10.1038/s41577-018-0005-7
[3] D. Vanderpool, B. Minh, et al. Primate phylogenomics uncovers multiple rapid radiations and ancient interspecific introgression. Plos Biology, vol. 18, no. 12, p. e3000954, 2020. https://doi.org/10.1371/journal.pbio.3000954
[4] H. Darusman, S. Mariya, et al. Spontaneous expression of the gene of ki67 and p53 in cynomolgus monkeys infected with papillomavirus. Veterinary World, p. 962-967, 2022. https://doi.org/10.14202/vetworld.2022.962-967
[5] S. Mariya, F. Dewi, et al. Isolation and characterization of c-c chemokine ligand 7 (ccl7) in cynomolgus macaques. Hayati Journal of Biosciences, vol. 26, no. 3, p. 129, 2019. https://doi.org/10.4308/hjb.26.3.129
[6] J. Uli, C. Yong, et al. Rna sequencing (rna-seq) of lymph node, spleen, and thymus transcriptome from wild peninsular malaysian cynomolgus macaque (macaca fascicularis). Peerj, vol. 5, p. e3566, 2017. https://doi.org/10.7717/peerj.3566