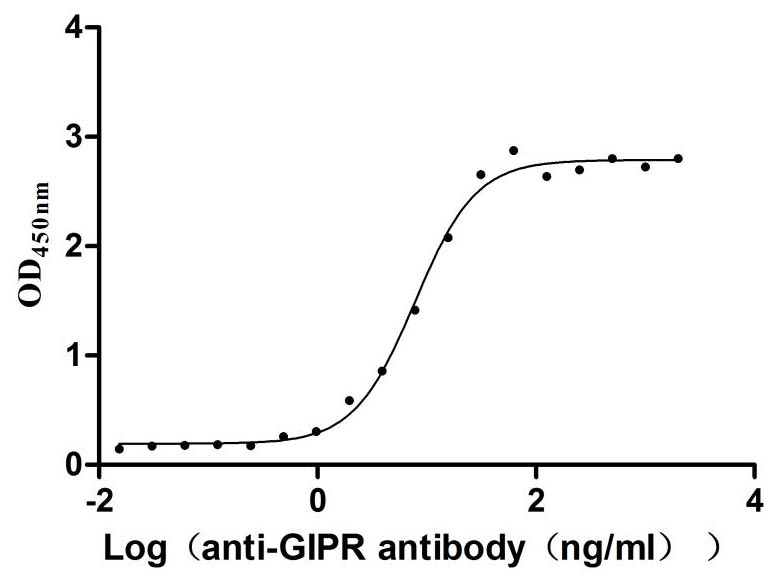
This recombinant rat Gipr protein, encompassing the extracellular domain (amino acids 19-135), is expressed in mammalian cells with a C-terminal 10×His tag, ensuring proper folding and post-translational modifications critical for receptor- ligand interactions. The recombinant Gipr protein exhibits exceptional purity (>95% by SDS-PAGE) and low endotoxin levels (<1.0 EU/μg, LAL method), meeting rigorous quality standards for functional assays. Validated via ELISA, it demonstrates specific binding to anti-mouse Gipr antibody (CSB-RA009438MA1MO) (EC50: 6.946–8.740 ng/mL at 2 μg/mL immobilization), confirming cross-species reactivity and structural integrity. The mammalian expression system preserves native conformational epitopes, which is essential for studying Gipr's role in glucose metabolism and insulin regulation. Provided as lyophilized powder, this preparation ensures stability and ease of reconstitution. It is ideal for investigating GIP-mediated signaling pathways, drug discovery for metabolic disorders, and receptor activation studies in diabetes research.
The Gipr is a critical G-protein coupled receptor (GPCR) that plays a significant role in the physiological response to glucose intake. Specifically, the Gipr mediates the effects of gastric inhibitory polypeptide (GIP), a hormone secreted by the K-cells of the intestine, which primarily stimulates insulin secretion from pancreatic β-cells in a glucose-dependent manner. This process is crucial in the regulation of blood glucose levels and overall glucose metabolism [1][2].
The Gipr gene in rats, as in many species, consists of multiple exons, with evidence suggesting that in rats, it is composed of 15 exons, leading to diverse receptor forms through alternative splicing [2]. Notably, the rat Gipr demonstrates a high degree of sequence homology to its human counterpart (approximately 80%), indicating conserved functional properties across species [3]. This high level of conservation allows researchers to study the Gipr function in rats as a proxy for human biology, especially in the context of metabolic diseases such as obesity and type 2 diabetes [4].
Recent studies have shown that Gipr expression is not restricted to the pancreas but is also prevalent in various regions of the central nervous system, including the hypothalamus, olfactory bulb, and hippocampus [5][6][7]. The expression of Gipr in the brain suggests that it may have roles beyond glucose metabolism, potentially influencing neuroendocrine activity, appetite regulation, and food intake. For instance, activation of hypothalamic Gipr-expressing neurons is linked to reduced food intake and regulation of energy balance [8]. This highlights the importance of Gipr in both metabolic and neurological contexts, emphasizing its potential as a therapeutic target in diseases like obesity and diabetes.
In terms of functional dynamics, Gipr is known to undergo variations in expression under different physiological and pathological conditions. For example, in models of type 2 diabetes, such as the Zucker diabetic fatty rat, Gipr mRNA and protein levels are significantly reduced in pancreatic islets, contributing to insulin secretion dysfunction [6][9]. Similarly, other models have indicated that alterations in Gipr expression can affect the insulinotropic action of GIP, leading to compromised glucose tolerance [10]. Moreover, receptor desensitization and downregulation in response to chronic stimulation can mimic functional antagonism, complicating therapeutic strategies aimed at enhancing Gipr functionality [4][11].
References:
[1] S. Sherman, J. Carr, D. Wang, M. O’Dorisio, T. O’Dorisio, & J. Howe. Gastric inhibitory polypeptide receptor (gipr) is a promising target for imaging and therapy in neuroendocrine tumors. Surgery, vol. 154, no. 6, p. 1206-1214, 2013. https://doi.org/10.1016/j.surg.2013.04.052
[2] C. Gaudin-Audrain, N. Irwin, et al. Glucose-dependent insulinotropic polypeptide receptor deficiency leads to modifications of trabecular bone volume and quality in mice. Bone, vol. 53, no. 1, p. 221-230, 2013. https://doi.org/10.1016/j.bone.2012.11.039
[3] C. Kelley, S. Decker, P. Silva, & J. Forrest. Gastric inhibitory peptide, serotonin, and glucagon are unexpected chloride secretagogues in the rectal gland of the skate (leucoraja erinacea). Ajp Regulatory Integrative and Comparative Physiology, vol. 306, no. 9, p. R674-R680, 2014. https://doi.org/10.1152/ajpregu.00531.2013
[4] E. Killion, M. Chen, et al. Chronic glucose-dependent insulinotropic polypeptide receptor (gipr) agonism desensitizes adipocyte gipr activity mimicking functional gipr antagonism. Nature Communications, vol. 11, no. 1, 2020. https://doi.org/10.1038/s41467-020-18751-8
[5] P. Higgins, R. Shade, et al. Central gip signaling stimulates peripheral gip release and promotes insulin and pancreatic polypeptide secretion in nonhuman primates. Ajp Endocrinology and Metabolism, vol. 311, no. 4, p. E661-E670, 2016. https://doi.org/10.1152/ajpendo.00166.2016
[6] F. Lynn, N. Pamir, E. Ng, C. McIntosh, T. Kieffer, & R. Pederson. Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty zucker rats. Diabetes, vol. 50, no. 5, p. 1004-1011, 2001. https://doi.org/10.2337/diabetes.50.5.1004
[7] A. Adriaenssens, E. Biggs, et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake., Cell Metabolism, vol. 30, no. 5, p. 987-996.e6, 2019. https://doi.org/10.1016/j.cmet.2019.07.013
[8] C. Smith, R. Patterson-Cross, et al. A comparative transcriptomic analysis of glucagon-like peptide-1 receptor- and glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus. Appetite, vol. 174, p. 106022, 2022. https://doi.org/10.1016/j.appet.2022.106022
[9] J. Nyberg, M. Anderson, et al. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. Journal of Neuroscience, vol. 25, no. 7, p. 1816-1825, 2005. https://doi.org/10.1523/jneurosci.4920-04.2005
[10] M. Gabe, W. Velden, et al. Enhanced agonist residence time, internalization rate and signalling of the gip receptor variant [e354q] facilitate receptor desensitization and long‐term impairment of the gip system. Basic & Clinical Pharmacology & Toxicology, vol. 126, no. S6, p. 122-132, 2019. https://doi.org/10.1111/bcpt.13289
[11] D. Gupta, M. Peshavaria, N. Monga, T. Jetton, & J. Leahy. Physiologic and pharmacologic modulation of glucose-dependent insulinotropic polypeptide (gip) receptor expression in β-cells by peroxisome proliferator–activated receptor (ppar)-γ signaling. Diabetes, vol. 59, no. 6, p. 1445-1450, 2010. https://doi.org/10.2337/db09-1655