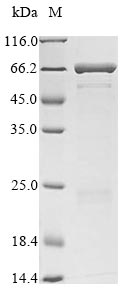
Recombinant Enterobacteria phage T4 Fibritin (wac) is produced in E.coli and contains the full-length mature protein, spanning amino acids 2-487. The protein includes an N-terminal 6xHis-SUMO tag that helps with purification and detection. SDS-PAGE analysis confirms the product reaches greater than 90% purity, which appears to make it suitable for various research applications.
Fibritin is a structural protein from Enterobacteria phage T4 that plays a critical role in phage assembly. This protein is involved in forming the phage tail—a component that seems crucial for both infectivity and viral stability. The unique characteristics of fibritin likely make it an important target for studies examining viral assembly mechanisms and protein-protein interactions in phage biology.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The T4 phage Fibritin is a structural protein that naturally forms trimers and functions as a molecular "glue" in phage tail assembly. While the SUMO tag may enhance solubility in E. coli, fibritin requires precise trimerization and proper folding for its native bioactivity (facilitating phage tail fiber attachment). E. coli can express viral proteins but may not replicate the exact oligomerization state without a proper cellular context. The large SUMO tag (≈100 aa) may sterically interfere with trimer formation. Without experimental validation of oligomerization and function, the protein cannot be assumed to be correctly folded or bioactive.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
The His-SUMO tag enables pull-down assays to identify binding partners (e.g., other phage proteins). However, if fibritin is misfolded or fails to trimerize, interactions may be non-physiological. Validate any identified partners using native fibritin from phage particles or confirm trimerization (e.g., via size-exclusion chromatography) before interaction studies.
2. Antibody Development and Immunological Studies
This application is suitable. The recombinant fibritin can serve as an immunogen for antibody production, as antibodies often recognize linear epitopes even in misfolded proteins. The high purity supports consistent immunization. However, antibodies generated may not recognize conformational epitopes specific to the native trimeric form. Validate antibody specificity against native T4 phage particles.
3. Biochemical Characterization and Stability Studies
This T4 phage Fibritin is suitable for basic biophysical analysis (e.g., circular dichroism for secondary structure, dynamic light scattering for aggregation state). However, data may not reflect native trimeric fibritin properties due to potential misfolding or tag interference. Use analytical ultracentrifugation or native PAGE to check trimer formation before structural conclusions.
4. ELISA-Based Binding Assays
Feasible only if the protein is properly folded and trimerized. Misfolded fibritin may not bind physiological partners (e.g., tail fibers) accurately, leading to false negatives. Confirm native structure and oligomerization before quantitative binding studies. The SUMO tag may sterically hinder binding sites.
Final Recommendation & Action Plan
Before using this recombinant fibritin for functional studies, validate its oligomerization and folding. First, analyze the oligomeric state via size-exclusion chromatography with multi-angle light scattering (SEC-MALS) or native PAGE to confirm trimer formation. If trimeric, test functionality in vitro (e.g., binding to T4 tail fibers). If inactive or monomeric, limit use to non-functional applications like antibody production (with validation against phage particles). For reliable interaction studies, consider expressing fibritin in a system that supports proper trimerization, or remove the SUMO tag to minimize steric interference. Always include appropriate controls (e.g., wild-type fibritin from phage) when studying binding or function.