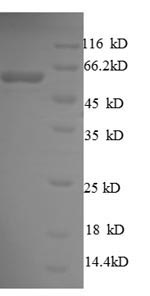
Amino acids 21-428 form the expressed segment for recombinant Escherichia coli (strain K12) surA. This surA protein is theoretically predicted to have a molecular weight of 61.1 kDa. This protein is generated in a e.coli-based system. The N-terminal 6xHis-SUMO tag was smoothly integrated into the coding gene of surA, which enables a simple process of detecting and purifying the surA recombinant protein in the following steps.
The SurA protein in Escherichia coli is a periplasmic chaperone that plays a critical role in the proper folding and stabilization of outer membrane proteins (OMPs). The periplasmic space is a challenging environment for protein folding due to oxidative conditions and the absence of ATP-driven folding machinery. SurA assists in the correct folding of OMPs, preventing their aggregation and facilitating their subsequent integration into the outer membrane. It acts at various stages of OMP biogenesis, aiding in the maintenance of their structural integrity. Additionally, SurA is involved in the quality control of OMPs, targeting misfolded proteins for degradation. This chaperone is crucial for bacterial fitness, membrane integrity, and resistance to environmental stresses. Research on SurA is vital for understanding the intricate mechanisms underlying bacterial protein folding and outer membrane biogenesis, providing potential targets for the development of antibacterial agents and drugs combating bacterial infections.