In a rapidly evolving field of biomedical research, why does a 50+ year-old technology like ELISA still dominate immunoassays?
Despite advancements in immunoassay technologies, ELISA remains the first choice for reliable and precise detection of proteins, antibodies, and hormones. But what makes ELISA the preferred method even when newer technologies like multiplex immunoassays promise more data in less time?
Table of Contents
1. What is ELISA?
ELISA is a widely used singleplex immunoassay technique for qualitative and quantitative analysis of antigens or antibodies in various samples. The fundamental principle of ELISA is based on the specific binding of an antigen to an antibody, which is then detected through an enzyme-labeled secondary antibody that produces a measurable signal, typically a color change, upon substrate addition.
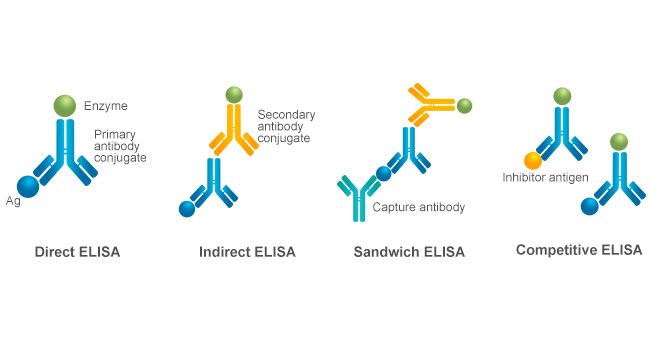
There are four major types of ELISA based on the configuration of the antibodies used and the detection method employed: direct ELISA, indirect ELISA, sandwich ELISA, and competitive ELISA. Each ELISA is tailored for specific research needs.
Figure 1. Major Four ELISA Types
Direct ELISA involves the enzyme-labeled antibody (or antigen) directly binding to the antigen (or antibody) immobilized on the surface of a microtiter plate. Then, the enzyme linked to the antibody (or antigen) reacts with its substrate to generate a visible signal that can be measured using a spectrophotometer. Direct ELISA is straightforward but may lack sensitivity compared to other types.
Indirect ELISA system involves a two-binding process of the primary antibody and enzyme-labeled secondary antibody. The primary antibody first binds to the immobilized antigen and then interacts with the enzyme-labeled secondary antibody, resulting in the development of color. This method enhances sensitivity but is more complex and costly due to the additional antibody [1].
Sandwich ELISA requires two antibodies specific to different epitopes of the same antigen. This format starts from the immobilization of a capture antibody on the microtiter plate. The antigen in the sample binds to the immobilized capture antibody and then is sandwiched with an enzyme-labeled detection antibody for color development. This method is highly sensitive and is commonly used for detecting proteins in complex samples.
Competitive ELISA describes a process in which the sample antigens compete with enzyme-labeled antigens for binding to a limited number of antibody sites [2]. The amount of signal produced is inversely proportional to the concentration of the target antigen in the sample. This method is particularly useful for small antigens or when the target is present in low concentrations [1,3].
2. What are Multiplex Immunoassays?
Multiplex immunoassays are advanced analytical techniques that can detect multiple analytes simultaneously from a single sample in a single run. The multiplexing approach significantly reduces the volume of samples required and the time taken for analysis, thus facilitating high-throughput screening and personalized medicine [4-6]. Multiplex assays have become increasingly popular in fields requiring comprehensive profiling of biological systems, like multiplex cytokine assay in immune responses or cancer biomarker analysis.
Multiplex immunoassays adopt traditional immunoassay principles. However, instead of using only one primary antibody, they utilize multiple primary antibodies, with each one identifying a different target.
Nearly all traditional immunoassays can be conducted in a multiplexed format. Multiplex immunoassays are classified into two categories according to the type of surface on which the antibodies or antigens are immobilized: planar assays and suspension microsphere assays.
In planar assay, multiple capture antibodies are immobilized on two-dimensional supports such as slides or microplate and probed with a sample, followed by the addition of chemiluminescent/fluorescent reporter-labeled detection antibodies. The chemiluminescent or fluorescent signals are detected using high-resolution scanner and are identified by xy coordinates [7]. The planar format is advantageous for high-density applications, enabling thousands of individual tests to be performed in parallel, thereby facilitating high-throughput screening [8,9].
In suspension immunoassays, the capture antibodies are immobilized on fluorophore-coded microspheres and then treated with sample, followed by the addition of fluorescently-tagged detection antibodies. Each microsphere accommodates a ‘sandwich’ containing the captured target analyte and the cognate reporter-conjugated detection antibody. Flow cytometry is used to detect the assay-specific fluorescent signals in the beads [10,11]. Bead-based immunoassays overcome mass transport limitations via active mixing throughout the liquid sample [12].
3. ELISA vs. Multiplex Immunoassays: Key Differences
ELISA and multiplex immunoassays are both pivotal techniques in the field of immunoassays, yet they exhibit distinct characteristics that influence their application in clinical and research settings.
| Feature |
ELISA |
Multiplex Immunoassays |
| Detection Capacity |
Single analyte detection per assay |
Simultaneous detection of multiple analytes per assay |
| Sample Volume |
Requires larger sample volume for multiple analytes, because each analyte requires a separate assay |
One single sample is used to measure multiple analytes |
| Sensitivity |
High sensitivity for individual analytes |
Sensitivity can be lower due to multi-analyte detection |
| Specificity |
Very high specificity due to individual optimization |
Moderate specificity; risk of cross-reactivity between multiple antibodies and analytes |
| Time Efficiency |
Time-consuming for multiple analytes, requires separate assays |
High efficiency, multiple targets detected in a single run |
| Cost and Equipment |
Simplicity of the technology means the required equipment is relatively inexpensive. Microplate readers are typically sufficient in most laboratories |
Higher initial cost for specialized equipment, but cost-effective for multi-analyte studies |
| Data Complexity and Analysis |
Simple, single readout per analyte; Data analysis is simple, typically involving a comparison to a standard curve |
Complex data with multiple readouts; requires specialized software and expertise |
| Ease of Use |
Easy to perform with simple procedure |
More complex protocols, requires technical expertise |
| Time to Results |
Takes longer for multi-analyte detection |
Shorter overall time for multi-analyte analysis |
| Versatility |
Suitable for a wide range of biomolecules |
Best suited for studies requiring broad profiling |
| Application |
Ideal for focused, single-analyte measurements |
Ideal for high-throughput, multi-analyte studies |
| Accuracy |
Very accurate and reliable |
May be less reliable for some low-abundance analytes, Accuracy can vary depending on the platform and assay optimization |
| Reproducibility |
Highly reproducibility due to its simplicity and focus on single analyte |
The complexity of measuring multiple analytes may result in variability and reduced reproducibility |
4. Why Does ELISA Remain the First Choice in Biomedical Research?
Despite the advent of multiplex assays, ELISA continues to be the preferred method for many immunoassays, especially in clinical diagnostics and focused research applications.
4.1 High Sensitivity and Specificity
ELISA can precisely detect and quantify individual analytes with high sensitivity and specificity. The use of highly selective antibodies for a single analyte ensures that results are accurate with minimal cross-reactivity. Whether in diagnostic labs measuring biomarkers like hormones or antibodies or in research settings investigating disease mechanisms, ELISA delivers reliable and reproducible results.
4.2 Simplicity and Accessibility
One of the major advantages of ELISA is its simplicity and ease of use. The procedures of ELISA are straightforward, making it easy to standardize across laboratories. The equipment required, such as microplate readers, is standard in most laboratories. Labs without advanced data analysis tools or bioinformatics support can still confidently interpret and report ELISA results.
4.3 Well-Established Protocols and Reproducibility
ELISA has been used for decades and has been thoroughly validated across many applications. It is trusted in both research and clinical settings for its reliability and accuracy. ELISA protocols are well-established, with a vast body of literature supporting their use. Researchers can rely on its reproducibility across experiments, with minimal variability between runs.
4.4 Cost-Effectiveness
When only one or two analytes need to be measured, ELISA is far more cost-effective than multiplex assays. The reagents and equipment required for ELISA are widely available and affordable, making it accessible to most labs. ELISA is a logical and economical choice for researchers working on a tight budget or small-scale studies.
4.5 Versatility Across Applications
ELISA can be adapted for various applications, from direct and indirect detection to sandwich and competitive formats. This flexibility allows ELISA to be used in diverse research areas, including immunology, infectious diseases, oncology, and pharmacology. Additionally, the ability to customize ELISA kits for specific research needs ensures that it remains a relevant and valuable tool in modern science.
Conclusion
While multiplex assays offer powerful capabilities for simultaneous multi-analyte detection, ELISA remains the go-to method for immunoassays, particularly when precision, simplicity, and cost-effectiveness are paramount. ELISA's unmatched sensitivity, specificity, versatility, and ease of use make it indispensable for many research and clinical applications. Multiplex assays have their place in high-throughput and comprehensive biomarker profiling studies, but for targeted, accurate quantification of individual analytes, ELISA continues to be the gold standard.
For researchers, the decision between ELISA and multiplex assays ultimately comes down to the specific needs of the study. However, when precision and reliability are the top priorities, ELISA remains the preferred option, ensuring its continued relevance in the evolving landscape of immunoassays.
CUSABIO can provide numerous premium ELISA kits capable of qualitative or quantitative detection of different targets in 15 research areas. They primarily use sandwich or competitive principles. Further, many ELISA kits have been cited in multiple prestigious high-impact publications, suggesting their excellent quality and validated performance.
Best-selling Products:
Mouse Tumor necrosis factor α, TNF-α ELISA KIT
CSB-E04741m
Human Tumor necrosis factor α, TNF-α ELISA KIT
CSB-E04740h
Further Reading:
11 tips for choosing your right ELISA kit
Do you Often Trouble in These Problems of ELISA?
Analysis of Factors Influencing ELISA Detection
What Factors Could Affect ELISA Results?
References
[1] K. Okada and K. Matsuo, Development of new antibodies and an elisa system to detect the potato alkaloids α-solanine and α-chaconine [J]. Foods, vol. 12, no. 8, p. 1621, 2023.
[2] Davies C (2013) Principles of competitive and immunometric assays (including ELISA) [J]. Immunoass Handb 29–59.
[3] M. Rasmussen, M. Dahl, et al. Evaluation of a competitive enzyme‐linked immunosorbent assay for measurements of soluble hla‐g protein [J]. Tissue Antigens, vol. 84, no. 2, p. 206-215, 2014.
[4] D. Li, H. Chiu, J. Chen, H. Zhang, & D. Chan, Integrated analyses of proteins and their glycans in a magnetic bead–based multiplex assay format [J]. Clinical Chemistry, vol. 59, no. 1, p. 315-324, 2013.
[5] W. Guo, H. Ding, C. Gu, et al. Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample [J]. Journal of the American Chemical Society, vol. 140, no. 46, p. 15904-15915, 2018.
[6] H. Ahsan and R. Ahmad, Multiplex technology for biomarker immunoassays [J]. 2021.
[7] P.J. Tighe, R.R. Ryder, I. Todd, L.C. Fairclough. ELISA in the multiplex era: potentials and pitfalls [J]. Proteom. Clin. Appl., 9 (2015), pp. 406-422.
[8] R. Wilson, A. Cossins, & D. Spiller. Encoded microcarriers for high‐throughput multiplexed detection [J]. Angewandte Chemie, vol. 45, no. 37, p. 6104-6117, 2006.
[9] M. Miller and Y. Tang. Basic concepts of microarrays and potential applications in clinical microbiology [J]. Clinical Microbiology Reviews, vol. 22, no. 4, p. 611-633, 2009.
[10] Ellington AA, Kullo IJ, Bailey KR, Klee GG. Antibody-based protein multiplex platforms: technical and operational challenges [J]. Clin Chem. 2010 Feb;56(2):186-93.
[11] Christine F. Markwalter, Keersten M. Ricks, et al. Simultaneous capture and sequential detection of two malarial biomarkers on magnetic microparticles [J]. Talanta Volume 161, 1 December 2016, Pages 443-449.
[12] J. Chou, J. Wong, et al. McDevitt. Porous bead-based diagnostic platforms: bridging the gaps in healthcare [J]. Sensors, 12 (2012), pp. 15467-15499.
CUSABIO team. ELISA vs. Multiplex Immunoassays: Why ELISA Remains the Gold Standard among Immunoassays. https://www.cusabio.com/c-21191.html




Comments
Leave a Comment