Lately, Supercede Therapeutics announced a $100 million partnership with Fudan University and East China Normal University to develop ActRII inhibitors for treating muscle atrophy, potentially in combination with GLP-1 drugs. Besides, Eli Lilly's acquisition of Versanis Bio has given them Bimagrumab, an ActRIIA/B monoclonal antibody with promising results in phase 2 trials for weight loss. More surprisingly, Merck's Sotatercept, a first-of-its-kind ACVR2A-Fc fusion protein, received FDA approval in March 2024 for Pulmonary Arterial Hypertension (PAH) treatment. These developments highlight the broad therapeutic potential of ActRII (mainly including ACVR2A and ACVR2B) in the pharmaceutical industry.
1. The Background of ACVR2A and ACVR2B
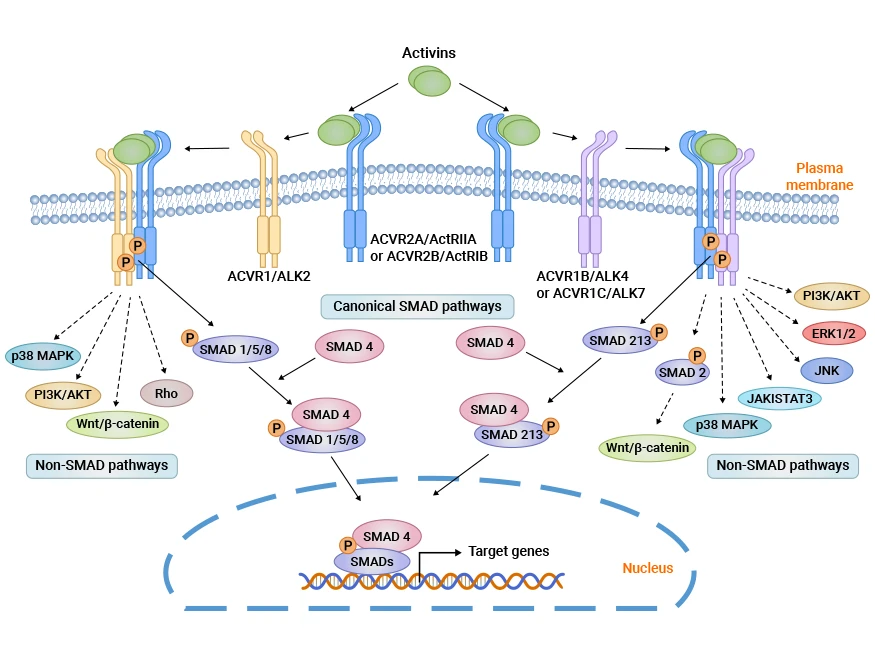
ActRIIA/ACVR2A and ActRIIB/ACVR2B, part of the TGF-β receptor superfamily, act as activin A type II receptors, crucial in cell growth, differentiation, and tissue repair. These membrane proteins, equipped with ligand-binding and kinase domains, trigger the activation of Smad2/3 and MAPK signaling pathways. This activation significantly impacts muscle mass, fat metabolism, and embryonic development. Though ActRIIA and ActRIIB share functions, their distinct tissue expression and ligand affinities enable specialized roles. All in all, ActRIIA and ActRIIB play crucial roles in metabolism, making them prime targets for drugs aimed at combating muscle atrophy and metabolic diseases. Ongoing development of Bimagrumab and KER-012 highlights promising new therapeutic approaches in this field (Figure 1) [1-7].
Figure 1. ActRII activates the SMAD signaling pathway [7]
2. The Applications of ACVR2A and ACVR2B in Disease Research
2.1 Skeletal Muscle Atrophy
ACVR2A and ACVR2B stand out as pivotal receptors within the TGF-β family that play a central role in regulating myostatin, thus significantly influencing muscle growth. Targeting these receptors can significantly enhance muscle development and prevent wasting. A notable example is the use of a soluble ACVR2B receptor, which has been found to effectively increase muscle mass and strength, as well as to prevent muscle atrophy induced by chemotherapy. Bimagrumab, an antibody designed to target both ACVR2A and ACVR2B, has shown significant promise in clinical trials by improving patient mobility and increasing muscle mass. These developments suggest promising new avenues for treating muscle atrophy and related conditions [8].
2.2 Obesity and Type II Diabetes
Notably, inhibiting ACVR2A and ACVR2B pathways may reduce obesity, which is the excess fat, while simultaneously preserving muscle mass. This dual action presents a compelling strategy for treating obesity. Additionally, Bimagrumab, a monoclonal antibody designed to target these receptors, has demonstrated significant effectiveness in clinical trials involving obese and Type 2 diabetes patients. It not only reduces body fat but also increases muscle mass, leading to improved metabolic parameters. Importantly, the potential long-term benefits and safety of this treatment are subjects for ongoing research, which will provide deeper insights into its overall efficacy [9-10].
2.3 Pulmonary Arterial Hypertension (PAH)
KER-012 is a therapeutic candidate that targets Activin Receptor Type IIB/ActRIIB to inhibit the signaling of TGF-β ligands, including activin A and B. It is currently being tested in a Phase II clinical trial (NCT05975905) for pulmonary arterial hypertension (PAH). At the same time, Merck's Sotatercept offers new hope for PAH patients by restoring balance between activin and bone morphogenetic protein pathways. This fusion protein significantly improves exercise capacity, hemodynamic parameters, and quality of life, while also delaying disease progression. Additionally, Sotatercept not only dilates pulmonary arteries but also remodels vascular structures, indicating it could be a promising new treatment strategy for PAH [11-12].
3. Drug Development Trends for ACVR2A and ACVR2B
Currently, several preclinical and clinical studies are exploring the effects of antagonists or agonists of ACVR2A and ACVR2B receptors in disease treatment. For example:
Bimagrumab is a monoclonal antibody currently in Phase II trials for treating obese or overweight patients. It functions by blocking Type II activin receptors (ActRII, including ACVR2A and ACVR2B), inhibiting myostatin signaling, which suppresses muscle growth. This action promotes skeletal muscle development and enhances brown fat activity. Clinical trial results show that Bimagrumab reduces overall fat mass by an average of 20.5% and increases lean body mass by 3.6% over 48 weeks in patients with obesity and type 2 diabetes, while also improving blood glucose levels. These results highlight Bimagrumab's potential as a new treatment option for obesity and related metabolic disorders.
Sotatercept is a fusion protein that received FDA approval on March 26, 2024, for treating adult pulmonary arterial hypertension (PAH). It acts as an activin signaling inhibitor by fusing the extracellular domain of ActRIIA with the Fc region of an antibody, creating a ligand trap that inhibits downstream signaling pathways and restores the balance between pro- and anti-proliferative signals. In the Phase 3 STELLAR trial, Sotatercept significantly improved patients' walking distance and reduced pulmonary vascular resistance, demonstrating strong clinical efficacy and offering a new treatment option for PAH patients.
LAE-102 is a monoclonal antibody targeting ACVR2A, with potential applications in treating endocrine and metabolic diseases, cancers, and respiratory conditions. Currently in the research phase, LAE-102 is expected to be utilized for obesity, overweight, metastatic solid tumors, and non-small cell lung cancer. Developed by Laekna Therapeutics Co., Ltd., it is in Phase I clinical trials globally. This information indicates that LAE-102 has the potential to become a valuable treatment option across multiple disease areas in the future.
Drugs targeting ACVR2A and/or ACVR2B, such as HS-20106, KER-050, and ALG-801, are in various stages of clinical trials and modulate these receptors' signaling pathways through different mechanisms. This ongoing research highlights the significant therapeutic potential of ACVR2A and ACVR2B in treating muscle atrophy and metabolic disorders, as well as their promise for future clinical applications. As studies progress, scientists are expected to uncover more characteristics and uses of ACVR2A and ACVR2B activin receptors, further solidifying their role as important therapeutic targets and paving the way for new treatment options for related diseases.
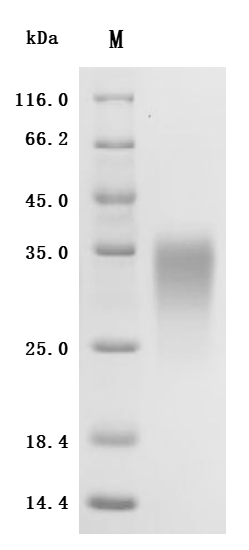
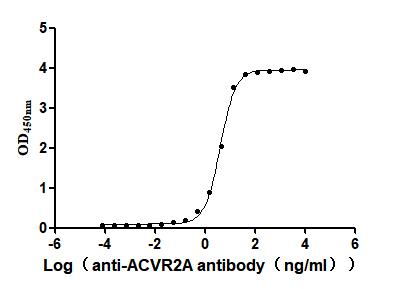
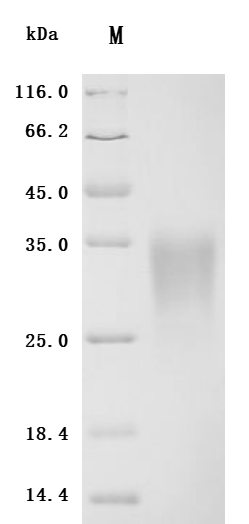
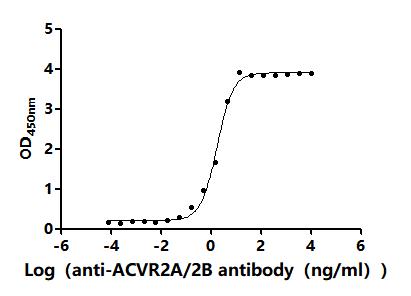
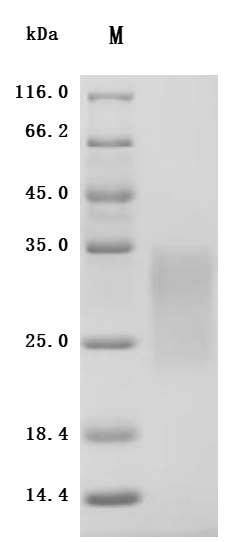
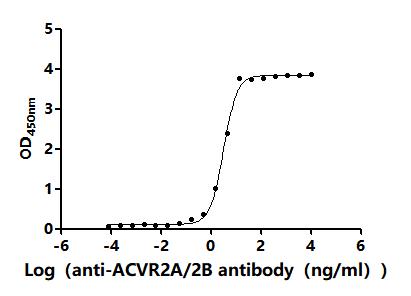
4. CUSABIO ACVR2A and ACVR2B Recombinant Proteins and Antibodies for Research Use
To assist pharmaceutical companies in their clinical research on ACVR2A and ACVR2B for applications in muscle growth, weight loss, and pulmonary arterial hypertension (PAH), CUSABIO has launched high-activity ACVR2A and ACVR2B protein products. These offerings aim to facilitate your exploration of the mechanisms and potential clinical value of these activin receptors.
References
[1] Mathews, L. S., et al. "Molecular cloning of the activin receptor, ActRII, and its role in the activin signaling pathway." Molecular Endocrinology 5.1 (1991): 56-66.
[2] Harrison, C. A., et al. "Identification of a second type II activin receptor, ActRIIb, which is a high-affinity receptor for activin." The Journal of Biological Chemistry 280.4 (2005): 3016-3024.
[3] Wu, Y., et al. "The role of TGF-β signaling in the regulation of muscle and fat metabolism." Frontiers in Physiology 12 (2021): 1-10.
[4] Keros Therapeutics. "KER-012: A novel therapeutic candidate targeting ACVR2A and ACVR2B for muscle wasting disorders." Clinical Trials (2023): 1-5.
[5] Kearney, C. J., et al. "Myostatin and activin signaling pathways in skeletal muscle: Implications for muscle wasting." Nature Reviews Endocrinology 15.3 (2019): 163-177.
[6] Keros Therapeutics. "Cibotercept (KER-012) in clinical trials for pulmonary arterial hypertension." Clinical Trials (2023): 1-5.
[7] Du, Ruochen, et al. "Activin receptors in human cancer: Functions, mechanisms, and potential clinical applications." Biochemical Pharmacology (2024): 116061.
[8] Lach-Trifilieff, E., et al. "An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy." Molecular and cellular biology 34.4 (2014): 606-618.
[9] Koncarevic, A., et al. "A novel therapeutic approach to treating obesity through modulation of TGFβ signaling." Endocrinology 153.7 (2012): 3133-3146.
[10] Suragani, R. N., et al. "Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis." Nature medicine 20.4 (2014): 408-414.
[11] Oshima, Y., et al. "Activin A and follistatin-like 3 determine the susceptibility of heart to ischemic injury." Circulation 119.14 (2009): 1969-1978.
[12] Yung, L. M., et al. "A selective transforming growth factor-β ligand trap attenuates pulmonary hypertension." American journal of respiratory and critical care medicine 194.9 (2016): 1140-1151.
CUSABIO team. ACVR2A and ACVR2B as Key Targets for Muscle Growth, Weight Loss, PHA in Drug Research!. https://www.cusabio.com/c-21198.html



Comments
Leave a Comment