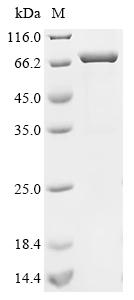
Recombinant Human Acetylcholine receptor subunit alpha (CHRNA1), Biotinylated, is produced in E. coli and spans amino acids 21-255 of the protein. It comes with an N-terminal MBP-tag and a C-terminal 6xHis-Avi tag, which makes it useful for different research applications. SDS-PAGE analysis shows the protein has a purity greater than 85%, which appears to be sufficient for most research purposes.
The Acetylcholine receptor subunit alpha (CHRNA1) serves as a crucial component of nicotinic acetylcholine receptors - integral membrane proteins that handle signal transduction across synapses. These receptors seem to play a key role in nerve signal transmission throughout the nervous system. They likely influence muscle contraction and various cognitive functions. Researchers extensively study CHRNA1 because of its involvement in neurological processes and its significance in how these receptors assemble and function.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The human CHRNA1 is a subunit of the nicotinic acetylcholine receptor, a complex transmembrane protein that requires correct folding, disulfide bond formation, glycosylation, and assembly with other subunits for bioactivity (e.g., ligand binding). E. coli, as a prokaryotic system, lacks the eukaryotic machinery for glycosylation and proper folding of transmembrane proteins. The large MBP tag (≈40 kDa) may improve solubility but can sterically hinder native folding, and the partial sequence lacks the transmembrane and intracellular domains essential for full function. The biotinylation via the Avi tag may be efficient, but without experimental validation (e.g., structural analysis or ligand binding assays), the protein is highly likely to be misfolded and inactive.
1. Protein-Protein Interaction Studies Using the Biotin-Streptavidin System
If the recombinant CHRNA1 is verified to be correctly folded (e.g., via circular dichroism or ligand binding assays), the biotin tag could enable interaction studies using streptavidin-coated surfaces. However, if misfolded, interactions may be non-physiological due to altered binding sites or tag interference. The MBP tag may further distort interactions. Validate any findings with full-length, native CHRNA1 from eukaryotic systems to ensure relevance.
2. Pull-Down Assays for Binding Partner Identification
This application is not reliable without folding validation. The dual tags (MBP and His) allow pull-down, but the partial fragment (21-255aa) may not mimic the extracellular domain's native structure. Misfolding could lead to false positives/negatives. Always confirm identified partners with full-length CHRNA1 expressed in mammalian cells.
3. ELISA-Based Binding Assays
Only feasible if the CHRNA1 protein is confirmed to have native-like folding and ligand-binding activity. If misfolded, ELISA results (e.g., for antibody or ligand binding) may be inaccurate. The biotin tag simplifies immobilization, but validate binding specificity with native receptors before quantitative applications like IC50 determination.
4. Antibody Development and Validation
This recombinant CHRNA1 can be used as an immunogen for antibody production, as antibodies often recognize linear epitopes even in misfolded proteins. The tags facilitate purification and screening. However, antibodies generated may not bind to native, glycosylated CHRNA1 in tissues or cells. Validate antibody specificity against full-length CHRNA1 from eukaryotic sources for applications like immunofluorescence or flow cytometry.
Final Recommendation & Action Plan
Before using this recombinant CHRNA1 for any functional application, prioritize experimental validation of its folding and bioactivity. Start with biophysical assays (e.g., circular dichroism to check for expected β-sheet-rich secondary structure) and a ligand binding assay (e.g., with α-bungarotoxin or acetylcholine) to confirm activity. If active, proceed with interaction or ELISA studies, but include controls like native CHRNA1. If inactive, limit use to antibody production, but rigorously validate antibodies against native protein. For reliable results, consider expressing CHRNA1 in a eukaryotic system (e.g., HEK293 cells) to ensure proper folding, glycosylation, and function. Avoid functional assays without prior activity confirmation.