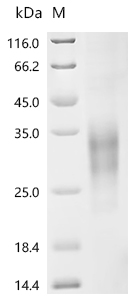
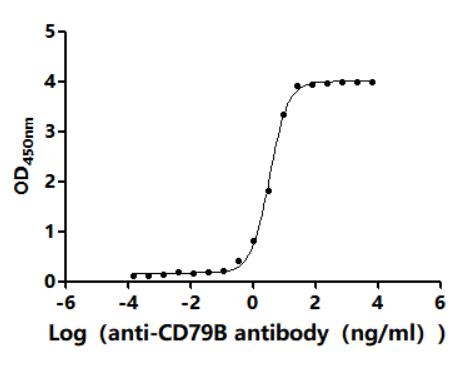
The human CD79B, amino acids Asp159-Arg143 with a C-terminal 10xHis-tag was co-expressed and purified as a human CD79B protein. The endotoxin of this recombinant human CD79B protein is less than 1.0 EU/ug as determined by the LAL method. This recombinant CD79B protein has been validated to be biologically active through a functional ELISA where it binds the anti-CD79B recombinant antibody (CSB-RA004958MA2HU), with the EC50 of 3.214-3.642 ng/mL.
The human CD79B protein is a crucial component of the B-cell receptor (BCR) complex, which plays a significant role in B-cell development and function. CD79B, along with its counterpart CD79A, forms a disulfide-linked heterodimer that associates with membrane immunoglobulin (mIg) to facilitate antigen recognition and signal transduction in B cells [1]. CD79B is primarily expressed on the surface of mature B cells and is absent in plasma cells, making it a valuable marker for identifying B-cell malignancies such as non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL) [2].
CD79B mainly transduces signals following antigen binding to the BCR. This signaling is essential for various B-cell activities, including differentiation, activation, and proliferation [3]. The BCR signaling pathway, which relies on CD79B, is critical for the formation of immune synapses and the regulation of B-cell migration [4]. Notably, aberrations in CD79B expression or function can lead to dysregulated BCR signaling, which is associated with immunosuppression in the tumor microenvironment [4].
Research has shown that mutations in the CD79B gene can impact its function and expression. Specific mutations in the immunoreceptor tyrosine-based activation motif (ITAM) of CD79B can lead to altered signaling capabilities, potentially contributing to the pathogenesis of B-cell lymphomas [5]. Additionally, the expression levels of CD79B can vary significantly among different B-cell malignancies, which has implications for therapeutic targeting using antibody-drug conjugates (ADCs) [6][7]. These ADCs are designed to selectively target CD79B on malignant B cells, thereby enhancing the efficacy of treatment while minimizing damage to normal cells [2].
The downregulation of CD79B has been observed in various hematological malignancies, suggesting that its expression is tightly controlled and can be disrupted in pathological conditions [8][9]. Understanding the regulatory mechanisms governing CD79B expression and function is critical for developing targeted therapies and improving patient outcomes in B-cell-related diseases.
References:
[1] L. Duncan, K. Webster, V. Gupta, S. Nair, & E. Deane. Molecular characterisation of the cd79a and cd79b subunits of the b cell receptor complex in the gray short-tailed opossum (monodelphis domestica) and tammar wallaby (macropus eugenii): delayed b cell immunocompetence in marsupial neonates, Veterinary Immunology and Immunopathology, vol. 136, no. 3-4, p. 235-247, 2010. https://doi.org/10.1016/j.vetimm.2010.03.013
[2] A. Herrera, M. Patel, J. Burke, R. Advani, B. Cheson, J. Sharman, et al. Anti-cd79b antibody–drug conjugate dcds0780a in patients with b-cell non-hodgkin lymphoma: phase 1 dose-escalation study, Clinical Cancer Research, vol. 28, no. 7, p. 1294-1301, 2022. https://doi.org/10.1158/1078-0432.ccr-21-3261
[3] D. Pu, D. Liú, C. Li, C. Chen, Y. Che, J. Lv, et al. A novel ten-gene prognostic signature for cervical cancer based on cd79b-related immunomodulators, Frontiers in Genetics, vol. 13, 2022. https://doi.org/10.3389/fgene.2022.933798
[4] Y. Wang, Y. Yang, Z. Zhao, H. Sun, D. Luo, L. Huttad, et al. A new nomogram model for prognosis of hepatocellular carcinoma based on novel gene signature that regulates cross-talk between immune and tumor cells, BMC Cancer, vol. 22, no. 1, 2022. https://doi.org/10.1186/s12885-022-09465-9
[5] B. Kloo, D. Nagel, M. Pfeifer, M. Grau, M. Düwel, M. Vincendeau, et al. Critical role of pi3k signaling for nf-κb–dependent survival in a subset of activated b-cell–like diffuse large b-cell lymphoma cells, Proceedings of the National Academy of Sciences, vol. 108, no. 1, p. 272-277, 2010. https://doi.org/10.1073/pnas.1008969108
[6] A. Polson, J. Calemine-Fenaux, P. Chan, W. Chang, E. Christensen, S. Clark, et al. Antibody-drug conjugates for the treatment of non–hodgkin's lymphoma: target and linker-drug selection, Cancer Research, vol. 69, no. 6, p. 2358-2364, 2009. https://doi.org/10.1158/0008-5472.can-08-2250
[7] M. Pfeifer, B. Zheng, T. Erdmann, H. Koeppen, R. McCord, M. Grau, et al. Anti-cd22 and anti-cd79b antibody drug conjugates are active in different molecular diffuse large b-cell lymphoma subtypes, Leukemia, vol. 29, no. 7, p. 1578-1586, 2015. https://doi.org/10.1038/leu.2015.48
[8] X. Huang, K. Takata, Y. Sato, T. Tanaka, K. Ichimura, M. Tamura, et al. Downregulation of the b‐cell receptor signaling component cd79b in plasma cell myeloma: a possible post transcriptional regulation, Pathology International, vol. 61, no. 3, p. 122-129, 2011. https://doi.org/10.1111/j.1440-1827.2010.02634.x
[9] D. Jovanovic, P. Djurdjevic, N. Andjelkovic, & L. Zivic. Possible role of cd22, cd79b and cd20 expression in distinguishing small lymphocytic lymphoma from chronic lymphocytic leukemia, Współczesna Onkologia, vol. 1, p. 29-33, 2014. https://doi.org/10.5114/wo.2013.38570