As crucial components of the B-cell receptor (BCR), CD79A and CD79B play a pivotal role in B-cell signal transduction and have garnered extensive attention in the field of blood cancer treatment. In recent years, significant progress has been made in the treatment of diffuse large B-cell lymphoma (DLBCL) with drugs targeting CD79B.
In December 2024, Roche’s Polatuzumab Vedotin (POLARIX study) announced 5-year follow-up data at the ASH annual meeting, demonstrating a significant improvement in progression-free survival (PFS) for DLBCL patients. Additionally, in February 2024, Hengrui Pharmaceutical’s CD79b ADC drug SHR-A1912 received Fast Track designation from the US FDA for the treatment of relapsed/refractory DLBCL patients.
This article will provide a detailed introduction to the structure, function, disease association, and drug development progress of CD79A and CD79B.
1. Structure and Function of CD79A and CD79B
1.1 Basic Structure of CD79A and CD79B
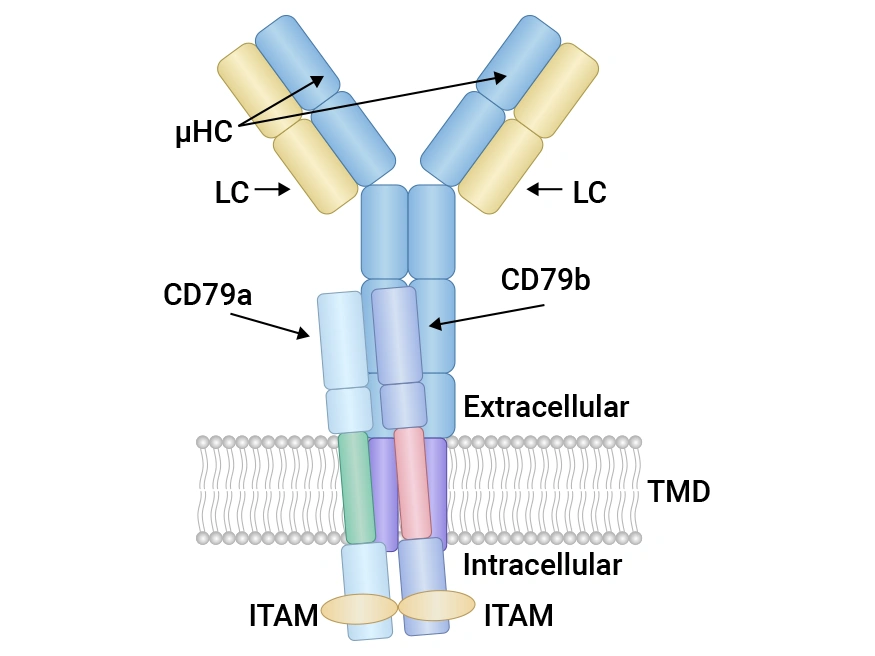
CD79A and CD79B are key components of the B-cell receptor (BCR) complex. CD79A (Igα) and CD79B (Igβ) are integral membrane proteins that form a heterodimeric complex with the immunoglobulin (Ig) molecule on the surface of B cells. Each protein consists of an extracellular domain, a transmembrane domain, and a cytoplasmic domain. The extracellular domain of CD79A and CD79B is responsible for interacting with the Ig molecule, while the cytoplasmic domain contains immunoreceptor tyrosine-based activation motifs (ITAMs) that are crucial for signal transduction. The heterodimeric structure of CD79A and CD79B is essential for the stability and functionality of the BCR complex. Crystal structure data have revealed that the extracellular regions of CD79A and CD79B interact through specific amino acids, forming a stable conformation that facilitates antigen binding and subsequent signal transduction [1].
Figure 1. Structure of CD79A and CD79B in the BCR.
Source: DOI: 10.3390/cancers15112881
1.2 Role of CD79A and CD79B in B-Cell Signal Transduction
CD79A and CD79B play a pivotal role in B-cell signal transduction. Upon antigen binding to the BCR, the tyrosine residues in the cytoplasmic domains of CD79A and CD79B are rapidly phosphorylated by Src-family kinases. This phosphorylation recruits signaling molecules containing SH2 domains, such as Syk kinase, initiating downstream signaling cascades. These cascades include the activation of phosphoinositide 3-kinase (PI3K), NF-κB, and mitogen-activated protein kinase (MAPK) pathways, which ultimately regulate B-cell activation, proliferation, and differentiation into antibody-secreting plasma cells. Studies in knockout mice have shown that the absence of CD79A or CD79B significantly impairs B-cell responses to antigens, highlighting their critical role in B-cell signaling [2].
1.3 Interaction and Signal Complex Formation of CD79A and CD79B
CD79A and CD79B form a stable heterodimer through disulfide bonds between their extracellular domains. This heterodimer interacts with the membrane-bound immunoglobulin (mIg) to form the BCR complex. The interaction between CD79A, CD79B, and mIg is specific, with different mIg isotypes exhibiting distinct binding modes. This specificity influences the efficiency and outcome of BCR signaling. Research has identified key amino acid residues at the interaction interface of CD79A and CD79B, and mutations in these residues can affect the stability of the complex and the efficiency of signal transduction. Cryo-electron microscopy studies have provided detailed insights into the interaction patterns between CD79A, CD79B, and mIg [3].
2. Mechanism of CD79A and CD79B in B-Cell Signal Transduction
2.1 Signal Amplification Mechanism Mediated by CD79A and CD79B
CD79A and CD79B mediate a powerful signal amplification mechanism. Following phosphorylation of their cytoplasmic tyrosine residues, CD79A and CD79B recruit multiple Syk kinase molecules. Syk kinase further phosphorylates downstream signaling molecules, such as phospholipase C-γ2 (PLC-γ2) and PI3K, initiating a cascade of events that amplify the initial antigen recognition signal. This amplification enhances the B-cell response to weak antigen stimuli, ensuring effective immune activation. In vitro studies have demonstrated that activation of the CD79A/CD79B signaling pathway results in significantly elevated phosphorylation levels of downstream signaling molecules, confirming the signal amplification effect [4].
2.2 Interaction of CD79A and CD79B with Other Signaling Pathways
CD79A and CD79B interact with several other important signaling pathways, including the PI3K-AKT and MAPK pathways. These interactions regulate B-cell proliferation, survival, and metabolism. PI3K, recruited to the CD79A/CD79B complex, activates AKT, which phosphorylates downstream target proteins, promoting B-cell growth and survival. CD79A and CD79B also influence the MAPK pathway, affecting the activity of transcription factors and regulating the expression of genes associated with B-cell activation and proliferation. This complex interplay forms a sophisticated signaling regulatory network that collectively modulates B-cell immune responses. Aberrant activation of the CD79A/CD79B signaling pathway in certain tumor cells can lead to excessive activation of the PI3K-AKT and MAPK pathways, promoting tumor cell proliferation and survival [5].
2.3 Key Role of CD79A and CD79B in B-Cell Activation and Proliferation
CD79A and CD79B are essential for B-cell activation and proliferation. They activate signaling pathways that lead to the activation of transcription factors such as NF-κB and AP-1. These transcription factors bind to target gene promoters, initiating the transcription of genes related to B-cell activation and proliferation. Studies have shown that inhibiting the function of CD79A or CD79B significantly suppresses B-cell activation and proliferation. In diffuse large B-cell lymphoma (DLBCL) cell lines, knockdown of CD79B expression markedly reduces cell proliferation and causes cell cycle arrest at specific stages [6].
3. Association of CD79A and CD79B with Diseases and Clinical Applications
3.1 Association of CD79A and CD79B with Tumor Development
CD79A and CD79B are closely associated with the development of various B-cell malignancies. In DLBCL, mutations in CD79B are common, particularly in the activated B-cell-like (ABC) subtype. These mutations lead to abnormal activation of the BCR signaling pathway, providing tumor cells with advantages in proliferation, survival, and drug resistance. In ABC-DLBCL patients, hotspot mutations in CD79B can maintain chronic BCR signaling, promoting tumor cell growth and invasion. Although mutations in CD79A are relatively rare, they have been detected in some lymphomas and contribute to tumor development. Research has shown that mutations in CD79A can affect its interaction with downstream signaling molecules, leading to abnormal signal transduction [7].
3.2 Role of CD79A and CD79B in Autoimmune Diseases
CD79A and CD79B also play significant roles in autoimmune diseases, such as systemic lupus erythematosus (SLE). In SLE patients, B cells are in a state of overactivation, with upregulated expression of CD79A and CD79B. The associated signaling pathways are excessively activated, leading to abnormal B-cell proliferation and differentiation, resulting in the secretion of large amounts of autoantibodies, causing tissue damage and organ dysfunction. Studies have shown that inhibiting the CD79A/CD79B signaling pathway can alleviate symptoms in SLE mouse models, suggesting that they may be potential therapeutic targets for autoimmune diseases. Elevated phosphorylation levels of CD79A and CD79B in peripheral blood B cells from SLE patients correlate with disease activity [8].
3.3 Potential of CD79A and CD79B as Biomarkers
Given the abnormal expression and functional changes of CD79A and CD79B in tumors and autoimmune diseases, they have significant potential as biomarkers. In tumor diagnosis, detecting the expression levels, mutation status, and other parameters of CD79A and CD79B in tumor tissues or blood can aid in early diagnosis, disease monitoring, and prognosis assessment. In DLBCL patients, detecting the mutation status of CD79B can help physicians determine patient prognosis and select more appropriate treatment strategies. In autoimmune diseases, monitoring changes in the expression of CD79A and CD79B can reflect disease activity, providing important information for the development of personalized treatment plans. Research has shown that detecting the mRNA levels of CD79A and CD79B in blood can effectively distinguish SLE patients from healthy controls [9].
4. Progress in Drug Development Targeting CD79A and CD79B
Targeting CD79A and CD79B has become a research hotspot in the biomedical field due to their critical roles in disease development. Currently, several drugs targeting CD79A and CD79B are in various stages of development, including monoclonal antibodies, antibody-drug conjugates (ADCs), and chimeric antigen receptor T-cell (CAR-T) therapies. These drugs are primarily aimed at B-cell lymphomas, with some entering clinical trials. The following table summarizes the progress of some drug development:
CD79A
| Target |
Drug |
Drug Type |
Indications |
Research Institution |
Highest Development Stage |
| CD20 x CD79A |
bbT-369 |
Autologous CAR-T |
Refractory B-cell lymphoma |
Diffuse large B-cell lymphoma | Regeneron Pharmaceuticals, Inc. |
Phase 1/2 |
| CD79A |
CN116731161 |
Monoclonal Antibody |
Tumor |
Nankai University |
Drug Discovery |
| CD79A x Protease |
WO2022214108 |
Fusion Protein |
Male Urogenital System Diseases | Skin and Musculoskeletal Diseases | Vascular Diseases |
Peking University First Hospital | Northwestern University |
Drug Discovery |
CD79B
| Target |
Drug |
Drug Type |
Indications |
Research Institution |
Highest Development Stage |
| CD79B x Tubulin |
Polatuzumab Vedotin |
ADC |
Refractory Lymphoma | Diffuse large B-cell lymphoma |
Hoffmann-La Roche, Inc. | Roche Holding AG | BSP Pharmaceuticals SpA |
Approved |
| CD32B x CD79B |
PRV-3279 |
Bispecific Antibody |
Systemic Lupus Erythematosus |
Huadong Pharmaceutical Co., Ltd. |
Phase 2 |
| CD79B |
SHR-A1912 |
ADC |
Lymphoma | B-cell Lymphoma |
Shanghai Hengrui Pharmaceutical Co., Ltd. |
Phase 2 |
| CD19 x CD79B |
CD19/79B 4SCAR-T (Shenzhen Geno-Immune Medical Institute) |
Autologous CAR-T |
B-cell Malignancies |
Shenzhen Geno-Immune Medical Institute |
Phase 1/2 |
| CD79B |
Anti-CD79B CAR-T (The Affiliated Hospital of Xuzhou Medical University) |
Autologous CAR-T |
B-cell Malignancies |
The Affiliated Hospital of Xuzhou Medical University |
Phase 1 |
| CD79B |
Anti-CD79B CAR-T (Wuhan Bio-Raid) |
Autologous CAR-T |
Hematological Tumors |
Wuhan Bio-Raid Biotechnology Co., Ltd. |
Phase 1 |
| CD79B |
BioLink018 |
Antibody |
Non-Hodgkin Lymphoma |
Protheragen, Inc. |
Phase 1 |
| CD19 x CD79B |
CD79b-19 CAR T Cells (Massachusetts General Hospital) |
Autologous CAR-T |
Follicular Lymphoma | High-grade B-cell Lymphoma |
The General Hospital Corp. |
Phase 1 |
| CD20 x CD3 x CD79B |
JNJ-80948543 |
Tri-specific T-cell Engager |
B-cell Leukemia | B-cell Lymphoma |
Janssen Research & Development LLC |
Phase 1 |
| CD79B |
JV-213 |
Autologous CAR-T |
B-cell Lymphoma |
The University of Texas MD Anderson Cancer Center |
Phase 1 |
| CD79B x Tubulin |
NBT-508 |
ADC |
Relapsed Transformed B-cell Non-Hodgkin Lymphoma | Refractory Mature B-cell Non-Hodgkin Lymphoma |
East China Pharmaceutical Co., Ltd. | Shanghai New Concept Biomedical
Technology Co., Ltd. |
Phase 1 |
| CD79B |
CD79b CAR-T Cell Therapy (Yake Biotechnology) |
Autologous CAR-T |
B-cell Lymphoma | Refractory B-cell Acute Lymphoblastic Leukemia |
Zhejiang University |
Early Phase 1 |
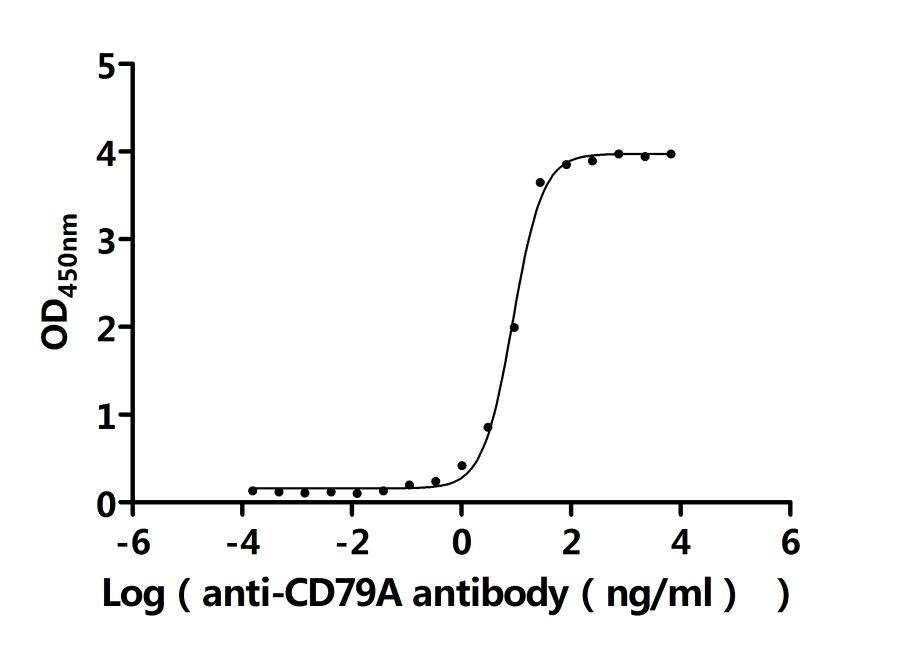
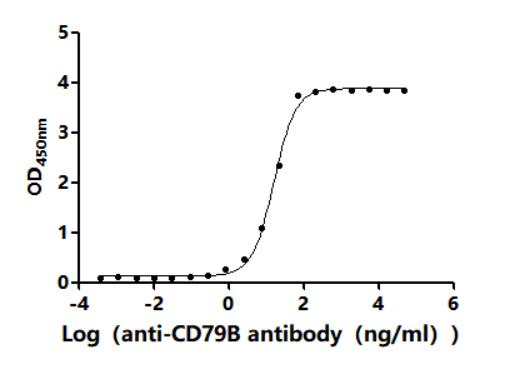
5. Recommended Products for CD79A and CD79B Research
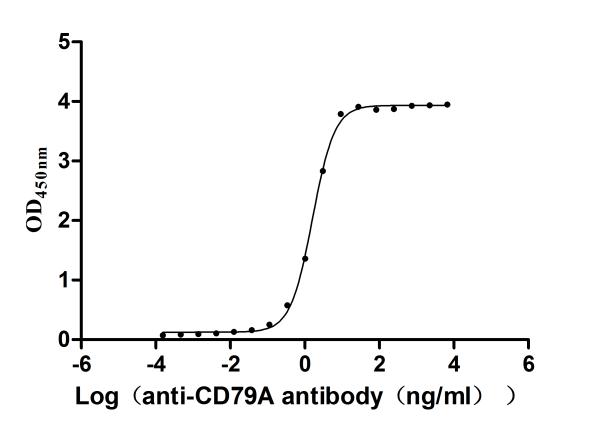
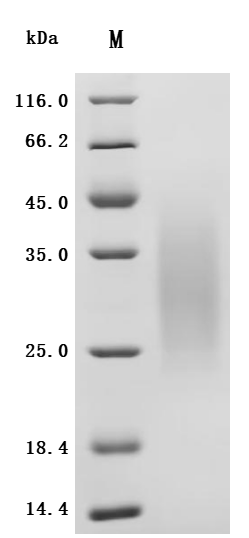
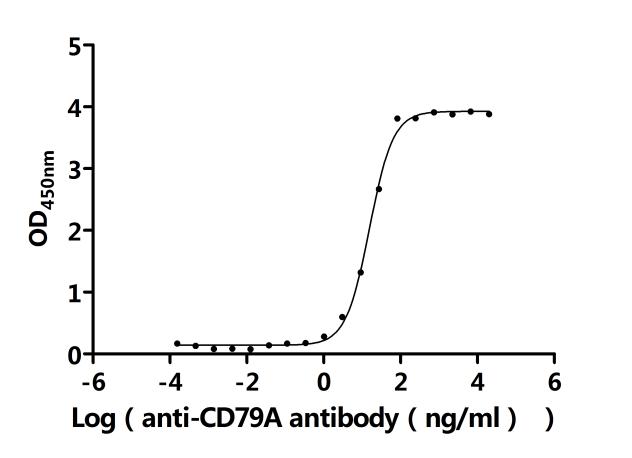
To facilitate your drug development efforts, CUSABIO has launched highly active recombinant CD79A and CD79B proteins and antibodies to support target mechanism exploration and potential clinical value research.
● CD79A Recombinant Protein
● CD79B Recombinant Protein
● CD79A & CD79B Recombinant Antibody
Click on CD79A and CD79B to view all related products.
References
[1] Chu PG, Arber DA. CD79: A review. Appl Immunohistochem Mol Morphol. 2001;9(2):97-106.
[2] Dong Y, Pi X, Bartels-Burgahn F, et al. Structural principles of B cell antigen receptor assembly. Nature. 2022;612(7938):156-161.
[3] Visco C, Tanasi I, Quaglia FM, et al. Oncogenic Mutations of MYD88 and CD79B in Diffuse Large B-Cell Lymphoma and Implications for Clinical Practice. Cancers. 2020;12(10):2913.
[4] Tkachenko A, Kupcova K, Havranek O. B-Cell Receptor Signaling and Beyond: The Role of Igα (CD79a)/Igβ (CD79b) in Normal and Malignant B Cells. Int J Mol Sci. 2024;25(1):10.
[5] Puja Bhattacharyya, et al. Combination of High-Resolution Structures for the B Cell Receptor and Co-Receptors Provides an Understanding of Their Interactions with Therapeutic Antibodies. Cancers (Basel). 2023.
[6] Wilson W, Young R, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(10):922-926.
[7] Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109(10):3879-3884.
[8] Gordon M, Kagan H, Wall R, et al. Aberrant B cell receptor signaling from B29 (Igβ) gene mutations of chronic lymphocytic leukemia B cells. Proc Natl Acad Sci USA. 2000;97(11):5504-5509.
[9] Xu P, Shen R, Shi Z, et al. The Prognostic Significance of CD79B Mutation in Diffuse Large B-Cell Lymphoma: A Meta-analysis and Systematic Literature Review. Clin Lymphoma Myeloma Leuk. 2022;22(10):e1051-e1058.
CUSABIO team. CD79A and CD79B: Key Targets in B Cell Signaling Pathways and Therapeutic Potential. https://www.cusabio.com/c-21210.html




Comments
Leave a Comment