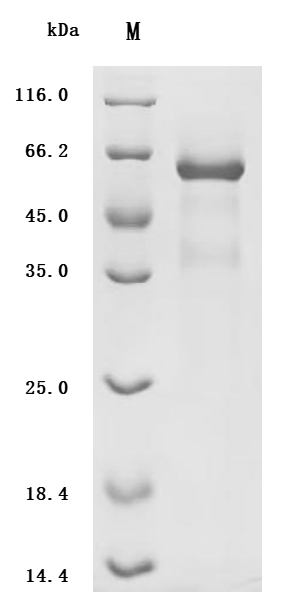
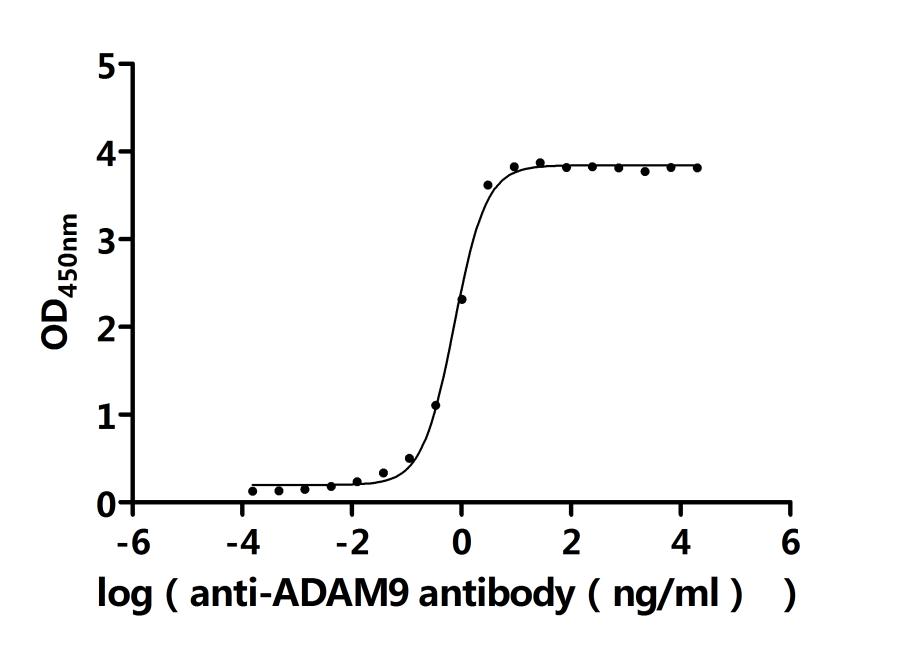
The recombinant ADAM9 is a recombinant human protein produced in a mammalian cell expression system. The target gene encoding the Ala29-Asp697 of the human ADAM9 is co-expressed with the C-terminal 10xHis-tag gene. Its purity is over 90% as determined by SDS-PAGE. Its endotoxin level is less than 1.0 EU/ug as determined by the LAL method. It has been validated to be biologically active in a functional ELISA where immobilized human ADAM9 at 2 μg/mL can bind the anti-ADAM9 recombinant antibody (CSB-RA618774MA1HU), with the EC50 of 0.9401-1.088 ng/mL.
Human ADAM9 (A Disintegrin and Metalloproteinase 9) is a member of the ADAM family of proteins, characterized by their metalloprotease and disintegrin domains. ADAM9 is widely expressed in various tissues and is known to be involved in the degradation of extracellular matrix (ECM) components, which is crucial for tumor cell invasion and migration [1][2][3][4].
ADAM9 is particularly notable for its involvement in cancer biology. Studies have shown that the expression of ADAM9 is significantly upregulated in several types of cancers, including prostate, lung, and gastric cancers. ADAM9 has been linked to increased invasive capacity and brain metastasis in non-small cell lung cancer (NSCLC) [2][5]. In prostate cancer, ADAM9 expression correlates with poor clinical outcomes and is associated with disease recurrence following treatment [6][7]. Furthermore, ADAM9 has been implicated in the regulation of tumor-stromal interactions, enhancing the motility of cancer cells [8].
The regulation of ADAM9 expression is influenced by various factors, including oxidative stress and hypoxia, which are common in the tumor microenvironment. Reactive oxygen species (ROS) have been identified as mediators that induce ADAM9 expression in prostate cancer cells, suggesting a link between stress responses and tumor progression [9][10].
Recent research has also highlighted the potential role of ADAM9 in viral infections, particularly in the context of SARS-CoV-2. It has been identified as a key factor that facilitates viral entry into cells, especially those with low ACE2 expression, indicating its relevance beyond cancer biology [11]. This dual role in both cancer and viral pathogenesis underscores the importance of ADAM9 as a target for therapeutic interventions.
References:
[1] R. Roychaudhuri, A. Hergrueter, F. Polverino, M. Laucho-Contreras, K. Gupta, N. Borregaard, et al. Adam9 is a novel product of polymorphonuclear neutrophils: regulation of expression and contributions to extracellular matrix protein degradation during acute lung injury, The Journal of Immunology, vol. 193, no. 5, p. 2469-2482, 2014. https://doi.org/10.4049/jimmunol.1303370
[2] Y. Shintani, S. Higashiyama, M. Ohta, H. Hirabayashi, S. Yamamoto, T. Yoshimasu, et al. Overexpression of adam9 in non-small cell lung cancer correlates with brain metastasis, Cancer Research, vol. 64, no. 12, p. 4190-4196, 2004. https://doi.org/10.1158/0008-5472.can-03-3235
[3] A. Chang, L. Lin, Y. Chen, P. Chen, S. Liu, H. Tai, et al. The adam9/wisp-1 axis cooperates with osteoblasts to stimulate primary prostate tumor growth and metastasis, International Journal of Biological Sciences, vol. 19, no. 3, p. 760-771, 2023. https://doi.org/10.7150/ijbs.77495
[4] R. Liu, F. Wang, Y. Guo, J. Yang, S. Chen, X. Gao, et al. Microrna-425 promotes the development of lung adenocarcinoma via targeting a disintegrin and metalloproteinases 9 (adam9), Oncotargets and Therapy, vol. Volume 11, p. 4065-4073, 2018. https://doi.org/10.2147/ott.s160871
[5] J. Zhang, J. Qin, N. Chen, W. Fu, B. Zhou, & A. He. High expression of a disintegrin and metalloproteinase-9 predicts a shortened survival time in completely resected stage i non-small cell lung cancer, Oncology Letters, vol. 5, no. 5, p. 1461-1466, 2013. https://doi.org/10.3892/ol.2013.1209
[6] S. Josson, C. Anderson, S. Sung, P. Johnstone, H. Kubo, C. Hsieh, et al. Inhibition of adam9 expression induces epithelial phenotypic alterations and sensitizes human prostate cancer cells to radiation and chemotherapy, The Prostate, vol. 71, no. 3, p. 232-240, 2011. https://doi.org/10.1002/pros.21237
[7] C. Pen, C. Liu, C. Lin, C. Lin, T. Hsieh, S. Josson, et al. Combined dynamic alterations in urinary vegf levels and tissue adam9 expression as markers for lethal phenotypic progression of prostate cancer, The Chinese Journal of Physiology, vol. 55, no. 6, p. 390-397, 2012. https://doi.org/10.4077/cjp.2012.baa075
[8] J. Wang, Y. Zhou, X. Fei, X. Chen, J. Yan, B. Liu, et al. Adam9 functions as a promoter of gastric cancer growth which is negatively and post-transcriptionally regulated by mir-126, Oncology Reports, vol. 37, no. 4, p. 2033-2040, 2017. https://doi.org/10.3892/or.2017.5460
[9] K. Shigemura, S. Sung, H. Kubo, R. Arnold, M. Fujisawa, A. Gotoh, et al. Reactive oxygen species mediate androgen receptor‐ and serum starvation‐elicited downstream signaling of adam9 expression in human prostate cancer cells, The Prostate, vol. 67, no. 7, p. 722-731, 2007. https://doi.org/10.1002/pros.20565
[10] J. Kim, H. Jeung, S. Rha, E. Yu, T. Kim, Y. Shin, et al. The effect of disintegrin–metalloproteinase adam9 in gastric cancer progression, Molecular Cancer Therapeutics, vol. 13, no. 12, p. 3074-3085, 2014. https://doi.org/10.1158/1535-7163.mct-13-1001
[11] I. Melano. A disintegrin and metalloproteinase domain 9 facilitates sars-cov-2 entry into cells with low ace2 expression, Microbiology Spectrum, vol. 11, no. 5, 2023. https://doi.org/10.1128/spectrum.03854-22