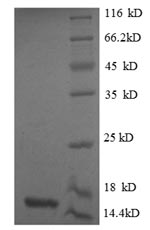
To produce recombinant human MSTN protein, the MSTN gene fragment (267-375aa) with the N-terminal 6xHis-tag gene is first cloned into an expression vector, which is subsequently introduced into E. coli cells. After the E. coli cells express the protein, it is subjected to affinity chromatography purification. Once the protein is isolated, its purity is evaluated using SDS-PAGE, greater than 90%. This high purity ensures the protein's effectiveness in the research associated with MSTN signal transduction.
Human myostatin (MSTN), also known as GDF-8, is a critical regulator of skeletal muscle growth and development. It belongs to the TGF-β superfamily and is primarily produced by myocytes. Myostatin functions as a myokine, exerting autocrine and paracrine effects that inhibit muscle cell proliferation and differentiation, thereby playing a significant role in muscle mass regulation [1][2][3].
The mechanism of action of myostatin involves its binding to the activin type II receptor (ActRIIB), which activates downstream signaling pathways, particularly the Smad pathway. This leads to the upregulation of cyclin-dependent kinase inhibitors such as p21, which subsequently inhibits cell cycle progression and promotes muscle atrophy [4][5]. Myostatin's inhibitory role is further underscored by studies demonstrating that blocking its signaling can lead to muscle hypertrophy and hyperplasia, indicating its function as a negative regulator of muscle mass [5][2][6].
References:
[1] A. Ryan and G. Li. Skeletal muscle myostatin gene expression and sarcopenia in overweight and obese middle‐aged and older adults, JCSM Clinical Reports, vol. 6, no. 4, p. 137-142, 2021. https://doi.org/10.1002/crt2.43
[2] M. Saitoh, J. Ishida, N. Ebner, S. Anker, J. Springer, & S. Haehling. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy, JCSM Clinical Reports, vol. 2, no. 1, p. 1-10, 2017. https://doi.org/10.17987/jcsm-cr.v2i1.37
[3] S. Lee and A. McPherron. Regulation of myostatin activity and muscle growth, Proceedings of the National Academy of Sciences, vol. 98, no. 16, p. 9306-9311, 2001. https://doi.org/10.1073/pnas.151270098
[4] C. McFarlane, G. Hui, et al. Human myostatin negatively regulates human myoblast growth and differentiation, Ajp Cell Physiology, vol. 301, no. 1, p. C195-C203, 2011. https://doi.org/10.1152/ajpcell.00012.2011
[5] Y. Chen, C. Gregory, M. Scarborough, R. Shi, G. Walter, & K. Vandenborne. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans, Physiological Genomics, vol. 31, no. 3, p. 510-520, 2007. https://doi.org/10.1152/physiolgenomics.00115.2006
[6] E. Kellum, H. Starr, et al. Myostatin (gdf-8) deficiency increases fracture callus size, sox-5 expression, and callus bone volume, Bone, vol. 44, no. 1, p. 17-23, 2009. https://doi.org/10.1016/j.bone.2008.08.126