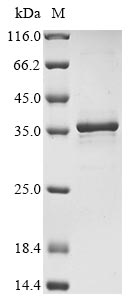
Recombinant Human Heat shock protein HSP 90-alpha (HSP90AA1) is produced in E. coli and contains amino acids 292 to 559, which represents a partial sequence of the protein. The protein carries an N-terminal 6xHis-tag that helps with purification and detection. The purity level exceeds 85% as verified by SDS-PAGE. This product is designed for research use only, though it appears to deliver reliable performance in laboratory settings.
HSP90AA1, commonly called Heat shock protein HSP 90-alpha, is a chaperone protein that may play a critical role in stabilizing and folding other proteins, particularly when cells face stress. It seems to be involved in various cellular processes, including protein maturation and degradation pathways. HSP90 has become a focal point in research because it's likely involved in maintaining cellular homeostasis, and there are potential implications in disease-related protein misfolding.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Human HSP90AA1 is a complex eukaryotic chaperone protein that requires precise folding, proper dimerization, and specific tertiary structure for its functional activity. The C-terminal domain (292-559aa) is involved in dimerization and client protein binding, but expressing this partial fragment in E. coli may not support correct folding due to the lack of eukaryotic chaperones and post-translational modifications. The N-terminal 6xHis-tag may cause steric interference with the protein's functional domains. While the fragment contains the C-terminal region, the probability of correct folding with functional bioactivity is low without experimental validation of dimerization capability and client binding.
1. HSP90 C-terminal Domain Structural Studies
This application carries a significant risk without folding validation. Structural studies require native conformation for meaningful insights. If correctly folded (verified through biophysical assays), the fragment may provide domain-specific structural data; if misfolded/unverified, results will not reflect the physiological structure and may be misleading.
2. Antibody Development and Validation
This application is highly suitable as antibody development relies on antigenic sequence recognition rather than functional protein folding. The defined fragment provides epitopes for generating C-terminal-specific antibodies. However, antibodies may not recognize conformational epitopes of full-length HSP90 in its native context.
3. Protein-Protein Interaction Studies
This application carries a high risk without functional validation. HSP90 interactions with clients or co-chaperones require precise tertiary structure and dimerization. If misfolded/unverified, pull-down assays may yield non-specific binding or tag-mediated artefacts rather than genuine physiological interactions.
4. Biochemical Characterization of Domain Function
Basic biophysical analysis can be performed but will not reflect native domain properties if misfolded. Techniques like circular dichroism spectroscopy or thermal stability assays can assess the fragment's properties, but results describe the recombinant construct rather than the physiological domain.
Final Recommendation & Action Plan
The E. coli-expressed HSP90AA1 C-terminal fragment with a His-tag may not be properly folded for functional studies due to the lack of eukaryotic folding machinery and the potential for steric interference. Begin with biophysical characterization (Application 4) to assess folding quality through techniques like size-exclusion chromatography (to check for dimerization) and circular dichroism spectroscopy. Applications 1 and 3 require rigorous validation of folding and dimerization capability before proceeding. Application 2 (antibody development) can proceed immediately. For reliable HSP90 research, use full-length protein expressed in eukaryotic systems (e.g., insect or mammalian cells) that support proper folding and dimerization, or implement refolding protocols with functional validation.