What Are NOD-like Receptors?
NOD (nucleotide-binding oligomerization domain)-like receptors, referred to as NLRs, are genetically conserved proteins that belong to cellular pattern recognition receptors (PRRs) protein family. They are important components in the innate immune system of mammals, regulating the immune response and inflammatory response.
The NLR family contains twenty-two human proteins and at least thirty-four mouse proteins that include a variable number of C-terminal leucine-rich repeats (LRRs), a central nucleotide-binding, oligomerization domain (NOD or NACHT), and an N-terminal protein interaction domain. The LRR is responsible for recognizing PAMPS (pathogen-associated molecular patterns) &DAMPS (danger-associated molecular patterns) and regulating NLR activity. NOD is a domain that exists only in members of the NLR family. It plays a critical role in the nucleotide-binding and self-oligomerization. The N-terminal protein interaction domain, also called the effector domain, contains pyrin domain (PYD), caspase activation, recruitment domain (CARD), and baculovirus inhibitor repeat domain (BIR). The effector domains exert their effects through isomorphic PYD-PYD or CARD-CARD interaction.
What Is the NOD-like Receptor Signaling Pathway?
The combination of NLRs and its homologous agonists initiates a signaling cascade that eventually leads to upstream regulation of the nuclear transcription factor Kappa B (NF-κB) and the production of pro-inflammatory cytokines. The process is called the NOD-like receptor signaling pathway.
The Function of NOD-like Receptor Signaling Pathway
The NLRs are responsible for detecting specific pathogen-associated molecules or host-derived damage signals in the cytosol and initiating the innate immune response. NOD-like receptors involve activation of multiple signaling pathways that are specific to a wide variety of microbial populations to control host-pathogen communication.
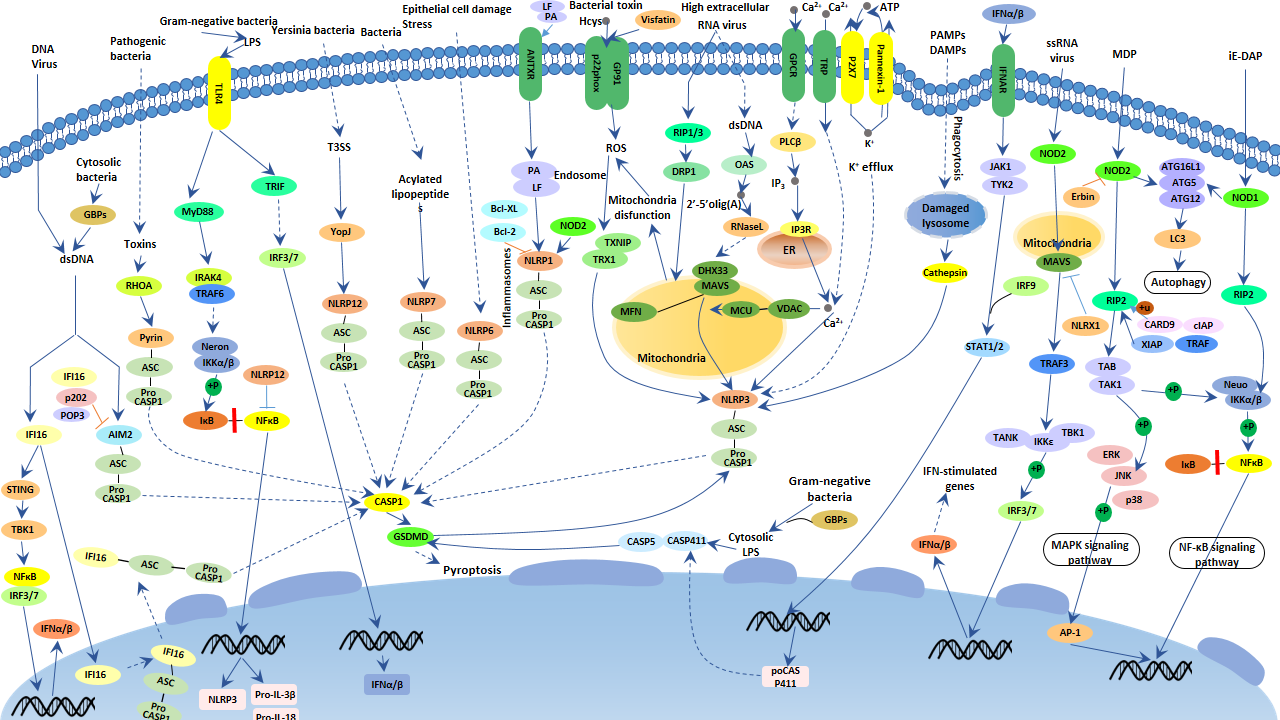
The Mechanism of NOD-like Receptor Signaling Pathway
NLRs are mostly located in the cytoplasm and are responsible for sensing intracellular danger signals. NLRs exert their functions through three types of signal transduction pathways, including the NF-κB pathway, the mitogen-activated protein kinase (MAPK) pathway, and the inflammasome pathway associated with IL-1β production.
NOD1 and NOD2 are the earliest discovered members of the NLR family. They can use the signal transduction of NF-κB and MAPK pathways to up-regulate a series of inflammatory products. Upon binding to their respective recognized ligands, NOD1 & NOD2 oligomerize at the NOD domain and recruit and activate RIP2 (receptor-interacting protein 2) through the CARD domain. Active RIP2 undergoes K63-linked polyubiquitination, which promotes the recruitment of downstream TAK1 and NEMO. The activation of TAK1 and NEMO causes phosphorylation of the key kinase IKKβ of the NF-κB pathway. IKKβ phosphorylation degrades IκB, leading to the NF-κB family transcription factors retained in the cytoplasm to enter the nucleus, which induces the expression of various inflammatory factors and chemokines. Although the molecular mechanism of the activation of the MAPK pathway by NOD1 and NOD2 is not as clear as the NF- κB pathway, it can be considered to be associated with the activation of RIP2 and TAK1.
Activation of NOD1 expressed in epidermal and mesothelial cells can cause the expression of chemokines, recruiting immune cells such as neutrophils to the site of infection. NOD1 and NOD2 molecules in epithelial cells can also express some anti-pathogenic peptides through the NF-κB pathway, preventing their passage from the epidermal defense line. Studies have shown that intestinal epithelial cells occur infectious enteritis if the mechanism mentioned above is missing. Additionally, activation of NOD1 and NOD2 can induce autophagy through interaction with Autophagy-related 16-like 1 (ATG16L1).
On the other hand, a different set of NLRs, including NLRP1, NLRP3, and NLRC4, induce caspase-1 activation through the assembly of multiprotein complexes called inflammasomes. The activated of caspase-1 regulates maturation of the pro-inflammatory cytokines IL-1β, IL-18 and drives pyroptosis. IL-1β release mediates the activation of the IL-1 receptor signaling pathway and myeloid differentiation factor (MyD88)-dependent nuclear factor kappa B, resulting in the transcription of cytokines such as IL-8 & macrophage inflammatory protein 2 (MIP2) and generating cascade amplification effect.
Although a clear connection between NOD1 and chronic inflammatory diseases has not been well-established, mutations in NOD2 have been linked to Crohn's disease and Blau syndrome.





