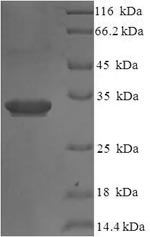
Recombinant Human Interleukin-36 receptor antagonist protein (IL36RN) is produced in E. coli with an N-terminal 6xHis-SUMO tag. The full-length protein spans amino acids 1-155 and appears to achieve a purity level greater than 90% as confirmed by SDS-PAGE. While designed for research use, this product may provide reliable reproducibility in experimental applications.
IL36RN seems to act as a crucial regulator within the immune system, inhibiting the activity of interleukin-36 cytokines. When it binds to the interleukin-36 receptor, IL36RN likely modulates inflammatory responses. This makes it an important focus in studies related to immune regulation and inflammation-associated pathways.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Human IL36RN is a cytokine that requires precise folding and disulfide bond formation for its functional activity in binding to the IL-36 receptor and antagonizing IL-36 signaling. The E. coli expression system cannot perform the necessary eukaryotic post-translational modifications (particularly proper disulfide bonding) that are critical for IL36RN's native conformation. The large N-terminal SUMO tag (∼15 kDa) may sterically interfere with the protein's receptor-binding interfaces. While the protein may be soluble, it is highly unlikely to achieve the correct folding needed for functional receptor antagonism.
1. Antibody Development and Validation Studies
This recombinant IL36RN serves as an excellent immunogen for generating antibodies against linear epitopes of human IL36RN. The full-length sequence ensures comprehensive coverage of the epitope. The SUMO tag facilitates purification and immunization procedures. However, antibodies may not efficiently recognize conformational epitopes on the native, properly folded IL36RN found in biological systems.
2. ELISA Development and Optimization
This protein is well-suited as a standard for quantitative ELISA to detect immunoreactive IL36RN levels. The assay depends on antibody binding to linear epitopes, so the protein's folding state is less critical. The His-SUMO tag enables consistent immobilization for reliable standard curve generation.
3. Biochemical Characterization and Stability Studies
This is the essential first step to assess the protein's physical properties. Techniques like size-exclusion chromatography can determine oligomeric state, while circular dichroism can analyze secondary structure content and thermal stability. These studies provide critical quality control data but characterize this protein itself, not native IL36RN.
Final Recommendation & Action Plan
The E. coli expression system is fundamentally unsuitable for producing a functional version of this complex cytokine, limiting its applications to non-functional uses. The protein can be reliably used for Application 1 (Antibody Development) and Application 2 (as an ELISA standard). Application 3 (Biochemical Characterization) should be prioritized to understand the protein's physical properties. Cytokine-receptor interactions require a precise tertiary structure that this bacterially expressed IL36RN cannot provide. For functional IL36RN studies, the only valid approach is to use the protein expressed in a eukaryotic system (e.g., mammalian cells) that can provide proper disulfide bond formation and folding.