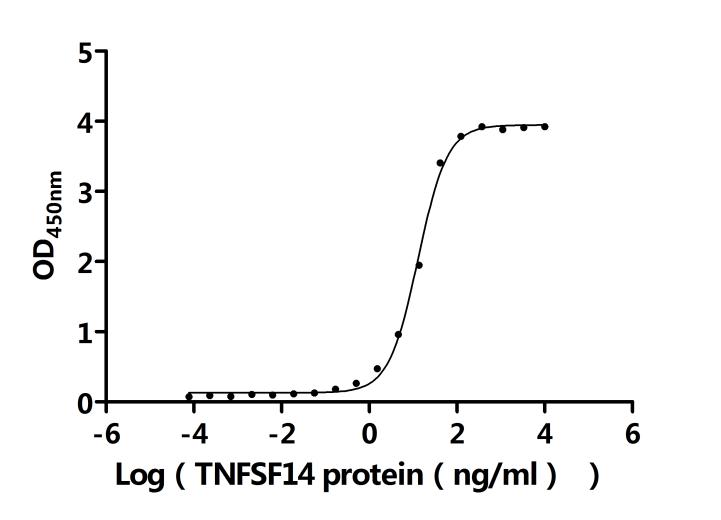
The recombinant Macaca fascicularis LTBR protein is an active, high-quality product produced in mammalian cells to preserve native folding and functional integrity. It comprises the amino acids 28 to 229 of the LTBR protein and includes a C-terminal 10xHis tag to facilitate purification and assay compatibility. This recombinant LTBR protein has been tested for endotoxin levels, which are maintained below 1.0 EU/µg as determined by the LAL method, ensuring suitability for sensitive biological applications. Functional activity is confirmed through ELISA, where immobilized LTBR at 2 μg/mL effectively binds human TNFSF14 (CSB-MP023991HUj7-B), with an EC50 ranging from 11.83 to 14.23 ng/mL. These characteristics support its use in receptor-ligand interaction studies, immune signaling research, and therapeutic target validation involving the LTBR-TNFSF14 axis.
The LTBR is a critical component of immune regulation and lymphoid organ development. In the context of Macaca fascicularis, commonly known as the cynomolgus monkey, understanding the function of LTBR can reveal insights into both primate immunology and potential applications in human health.
LTBR is known for its role in mediating lymphoid organogenesis, particularly during the development of secondary lymphoid tissues such as lymph nodes and spleen. This receptor interacts with lymphotoxin, a cytokine that is crucial for the organization of lymphoid tissues. Research has demonstrated that the signaling pathway activated by LTBR contributes significantly to the maintenance and proliferation of various immune cells, including T and B lymphocytes, which are essential for adaptive immunity [1]. In studies involving non-human primate models, including Macaca fascicularis, LTBR has been recognized for its involvement in regulating immune responses to pathogens, making it a potent candidate for studying immune interventions and therapies against infectious diseases like tuberculosis [2].
Further exploration of LTBR in cynomolgus monkeys has shown its implications in disease modeling. For instance, the dynamics of LTBR signaling can be applied to understanding the progression of infections, such as those caused by Mycobacterium tuberculosis, where the receptor's activity may influence granuloma formation, a hallmark of pulmonary tuberculosis [2]. Additionally, the utilization of cynomolgus monkeys in vaccine research against viruses like SARS-CoV-2 implicates LTBR in understanding immune memory and responsiveness in vaccine contexts [3][4].
The relevance of LTBR is further supported by its similarities across species, allowing for translational insights into human immune responses. Since cynomolgus monkeys share significant anatomical and immunological parallels with humans, findings regarding LTBR in these monkeys can be valuable benchmarks for developing therapeutic interventions in human health, particularly in immunology and vaccine development [4]. Various studies on LTBR have highlighted its role in disease progression and recovery, further elucidating its potential as a therapeutic target.
References:
[1] J. Flynn, H. Gideon, J. Mattila, & P. Lin. Immunology studies in non‐human primate models of tuberculosis. Immunological Reviews, vol. 264, no. 1, p. 60-73, 2015. https://doi.org/10.1111/imr.12258
[2] L. Hunter, S. Hingley‐Wilson, G. Stewart, S. Sharpe, & F. Salguero. Dynamics of macrophage, t and b cell infiltration within pulmonary granulomas induced by mycobacterium tuberculosis in two non-human primate models of aerosol infection. Frontiers in Immunology, vol. 12, 2022. https://doi.org/10.3389/fimmu.2021.776913
[3] C. Brady, T. Tipton, S. Longet, & M. Carroll. Pre-clinical models to define correlates of protection for sars-cov-2. Frontiers in Immunology, vol. 14, 2023. https://doi.org/10.3389/fimmu.2023.1166664
[4] J. Estes, S. Wong, & J. Brenchley. Nonhuman primate models of human viral infections. Nature Reviews Immunology, vol. 18, no. 6, p. 390-404, 2018. https://doi.org/10.1038/s41577-018-0005-7