Lymphotoxin β Receptor (LTBR), also known as Tumor Necrosis Factor Receptor Superfamily Member 3 (TNFRSF3), plays a crucial role in the immune system and is vital for maintaining immune balance and overall health. As research continues to deepen, its mechanisms in disease development have become increasingly clear, providing important theoretical basis for the treatment of related diseases. This article will discuss the structure, function, signal transduction pathways, immune regulatory role, disease associations, and drug development progress related to LTBR.
1. Structure and Function of LTBR
1.1 Structural Characteristics of LTBR
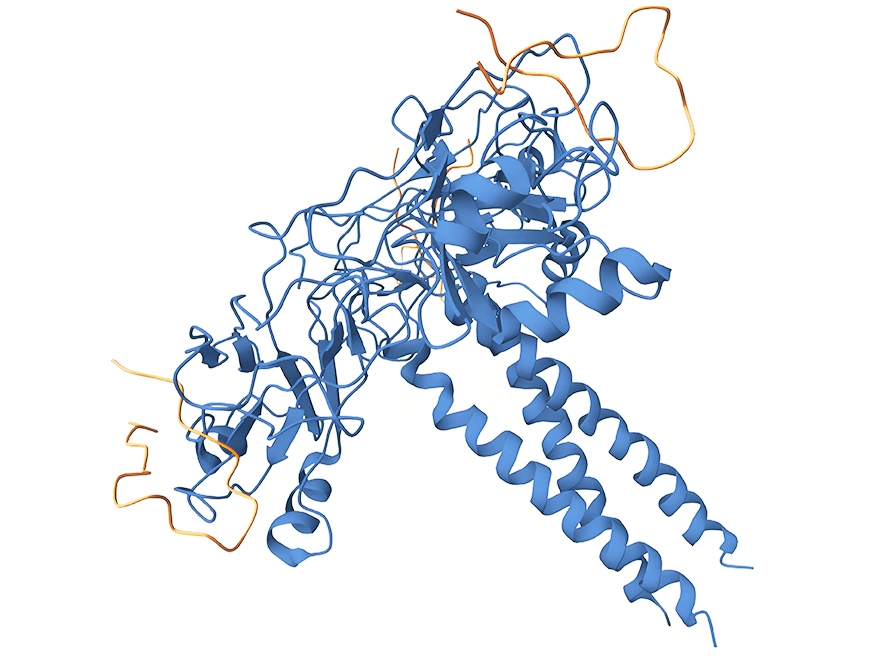
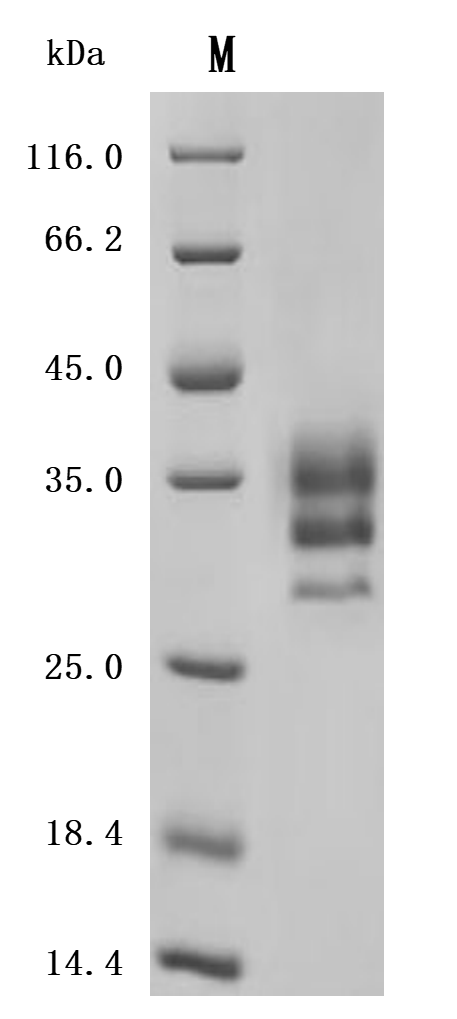
LTBR is a type I single-pass transmembrane protein [1] and a member of the Tumor Necrosis Factor Receptor (TNFR) family [2]. The fully glycosylated LTBR has a molecular weight of 61kDa, while the theoretical molecular weight without glycosylation is 47kDa [3]. Its cytoplasmic domain consists of 175 amino acids, with a proline-rich region near the cell membrane, enabling it to interact directly with Tumor Necrosis Factor Receptor-Associated Factors (TRAFs) and play a key role in cell signaling [4]. The LTBR protein structure includes an extracellular domain, transmembrane domain, and intracellular domain (Figure 1). The extracellular domain is responsible for ligand binding and recognition of specific signal molecules, while the intracellular domain initiates intracellular signaling pathways through interaction with TRAF proteins, thereby regulating cell biological behavior [5].
Figure 1: Structure of LTBR Protein
Source: https://www.uniprot.org/uniprotkb/P36941/entry
1.2 Expression and Distribution of LTBR
LTBR is expressed in various immune cells, such as fibroblastic reticular cells, endothelial cells, epithelial cells, and myeloid cells in lymphoid tissues [6]. Although not expressed in T cells, B cells, and NK cells, it has an indirect yet important impact on the function regulation of these cells.
2. The Role of LTBR in the Immune System
2.1 Regulation of Immune Cell Function by LTBR
LTBR is involved in processes such as cell apoptosis and cytokine release, making it an important participant in cell-to-cell communication and immune response regulation within the immune system [7]. During immune responses, LTBR regulates the release of cytokines, affecting the activation and migration of immune cells, and thus regulating the intensity and direction of immune responses. Studies have shown that activation of the LTBR signaling pathway can promote the proliferation and differentiation of T cells and B cells, enhancing immune responses [8].
2.2 Relationship between LTBR and Lymphoid Organ Development
LTBR plays an indispensable role in the development of secondary lymphoid organs [9]. During embryonic development, activation of the LTBR signaling pathway promotes the formation of lymphoid organ primordia and subsequently regulates the homing and differentiation of immune cells, enabling the normal development of lymph nodes, spleen, and other lymphoid organs. Additionally, LTBR is involved in the remodeling and maintenance of lymphoid tissues, which is crucial for the normal functioning of the immune system [10].
Figure 2: The LTβR Signaling Pathway in Lymphatic Endothelial Cell (LEC).
Source: doi:10.3390/cells10040747.[13]
3. LTBR Signal Transduction Pathways
3.1 Signal Transduction Mechanism of LTBR
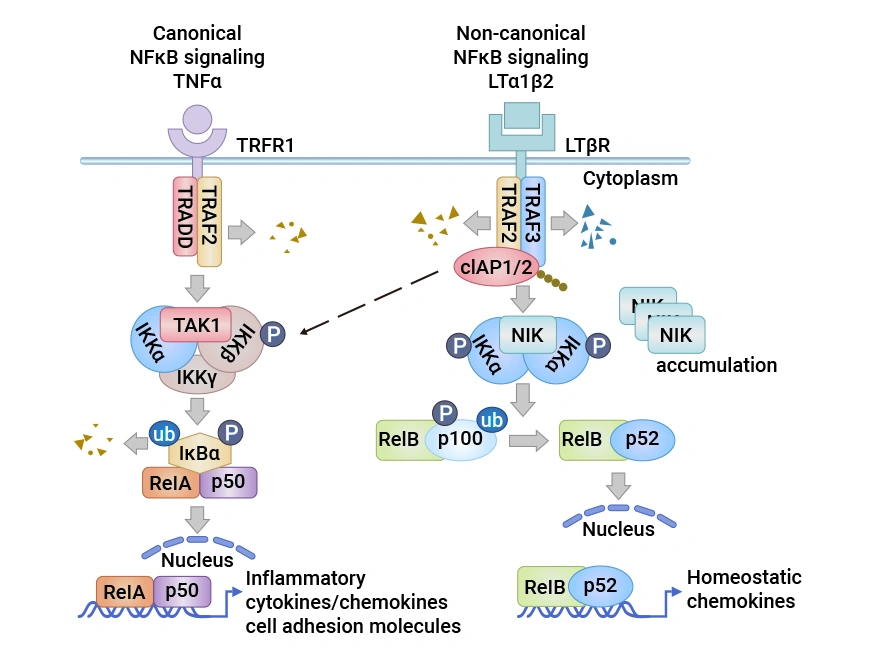
LTBR's signaling has pleiotropic functions, and binding to ligands triggers a series of complex signaling events. LTBR has two natural ligands, LTα1β2 and LIGHT (TNFSF14) [11]. LTα1β2 is a purely membrane-bound protein anchored by the LTβ subunit and binds only to LTBR; whereas LIGHT can bind to both LTBR and HVEM and exists in both membrane-bound and soluble forms. After ligand binding to LTBR, TRAF2 and TRAF3 are recruited to the LTBR complex [12].
3.2 Related Downstream Signaling Pathways
During the recruitment process, TRAF2 and TRAF3 are degraded by cIAP1/2, leading to the stabilization and accumulation of NF-κB inducing kinase (NIK) [13]. NIK and the IKKα complex are activated, promoting the phosphorylation of homodimeric IKKα. Ultimately, the p100 precursor bound to RelB is cleaved into p52, and the RelB-p52 heterodimer complex translocates to the nucleus, initiating chemokine gene transcription. Simultaneously, LTBR also activates IKKα/β phosphorylation and RelA/p50 nuclear translocation, triggering the transcription of inflammatory and cell adhesion molecule genes. These signaling pathways work together to regulate cell proliferation, survival, and the expression of immune-related molecules.
3.3 Signal Transduction in Cell Interactions Mediated by LTBR
LTα1β2 is mainly expressed on T cells and B cells, which lack LTBR expression, forming a unique signaling communication mode. This mode allows the LTα1β2-LTBR signal to play a unique role in the communication between lymphocytes and LTBR-bearing cells, coordinating the interactions between immune cells and the intensity of immune responses. For example, during antigen presentation, T cells interact with antigen-presenting cells expressing LTBR through LTα1β2 expression, promoting T cell activation and the initiation of immune responses [5].
4. LTBR and Immune Regulation
4.1 Role of LTBR in B Cell Activation and Proliferation
Although B cells do not express LTBR, its signaling has a significant impact on B cell activation and proliferation. The cytokines and chemokines it regulates can provide a suitable microenvironment for B cell activation and proliferation, promoting B cell antigen recognition and immune responses [7]. Studies have shown that in the absence of LTBR signaling, B cell activation and antibody production are significantly inhibited, indicating that LTBR plays a key supportive role in B cell immune responses [8].
4.2 Impact of LTBR on T Cell Function Regulation
LTBR also regulates T cell function. It can influence the differentiation direction of T cells, regulate their activation and proliferation, and modulate the secretion of cytokines by T cells, thus playing a key regulatory role in cell-mediated immunity [9]. During T cell differentiation, activation of the LTBR signaling pathway can promote the differentiation of Th1 and Th17 cells, enhancing cell-mediated immune functions [10].
4.3 Role of LTBR in Antibody Production and Immune Memory
LTBR is involved in regulating the differentiation of B cells into plasma cells during antibody production, promoting antibody synthesis. Moreover, LTBR plays a role in the formation and maintenance of immune memory, helping the immune system remember invading pathogens so that it can respond rapidly upon re-exposure [2]. Experimental data indicate that blocking LTBR signaling during the formation of immune memory leads to a reduction in the number and function of immune memory cells, affecting the body's resistance to reinfection [12].
5. Research Progress of LTBR in Diseases
5.1 Relationship between LTBR and Autoimmune Diseases
Research has found that the LTBR signaling pathway is associated with various autoimmune diseases [4]. In some autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, the LTBR signaling pathway may be abnormally activated or dysregulated, causing the immune system to mistakenly attack its own tissues, triggering inflammation and tissue damage [5]. In the synovial tissue of patients with rheumatoid arthritis, the expression level of LTBR is significantly increased, and the excessive activation of its related signaling pathway promotes the infiltration of inflammatory cells and the release of inflammatory factors, exacerbating joint inflammation and destruction [6].
5.2 Role of LTBR in Infectious Diseases
In infectious diseases, LTBR plays an important role in the body's resistance to pathogen infection. It can regulate the function of immune cells and enhance the body's ability to eliminate pathogens [7]. However, some pathogens may also exploit the LTBR signaling pathway to evade immune surveillance or overactivate immune responses, leading to immune pathological damage [8]. For example, during viral infections, viruses may interfere with the LTBR signaling pathway to suppress the activation and function of immune cells, thereby achieving immune evasion [9].
5.3 Correlation between LTBR and Tumor Immunity
In the tumor microenvironment, the role of LTBR has attracted much attention. On one hand, activation of the LTBR signaling pathway can induce the formation of tertiary lymphoid structures (TLS) [10]. TLS are aggregates of immune cells that can promote the influx of immune cells into the tumor site and enhance the body's anti-tumor immune responses [11]. Many studies have shown that the presence and the number and density of TLS within tumors are associated with a good prognosis in various cancer types. In melanoma patients, high infiltration of TLS within tumor tissue is related to extended patient survival and reduced recurrence rates [12]. On the other hand, tumor cells may also regulate the LTBR signaling pathway to suppress the function of immune cells, thereby achieving immune evasion [13]. Some tumor cells secrete inhibitory cytokines to interfere with the normal conduction of the LTBR signaling pathway, preventing immune cells from effectively recognizing and killing tumor cells [5].
6. Progress in LTBR-Related Drug Development
Based on the important role of LTBR in immune regulation and disease development, several antibody drug pipelines are currently under development, in the preclinical or drug discovery stages, with the main indication being tumors. Some of the drugs under development are listed below:
| Drug Name |
Drug Type |
Indications for Research |
Research Institutions |
Highest Research Stage |
| hCBE-11 |
Monoclonal Antibody |
Rectal Cancer |
Biogen, Inc. |
Preclinical |
| M-300 (Mestag) |
Bispecific Antibody |
Solid Tumors |
Mestag Therapeutics Ltd. |
Preclinical |
| CN117377687 |
Nanobody |
Solid Tumors |
Vlaams Instituut voor Biotechnologie - Flanders Institute | Université Catholique de Louvain | Vrije Universiteit Brussel |
Drug Discovery |
| WO2024211478 |
Cell Therapy |
Tumors | Genitourinary System Diseases |
Regeneron Pharmaceuticals, Inc. |
Drug Discovery |
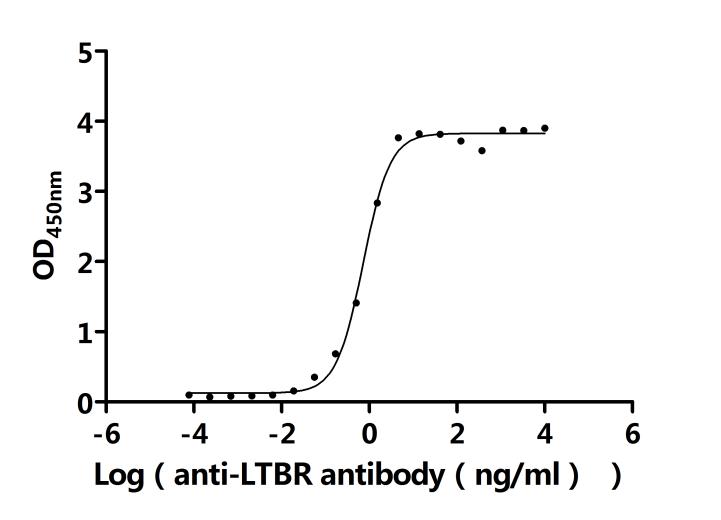
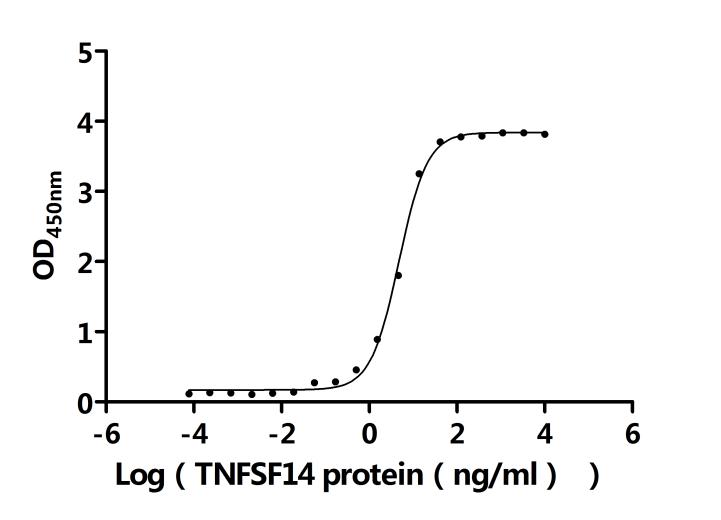
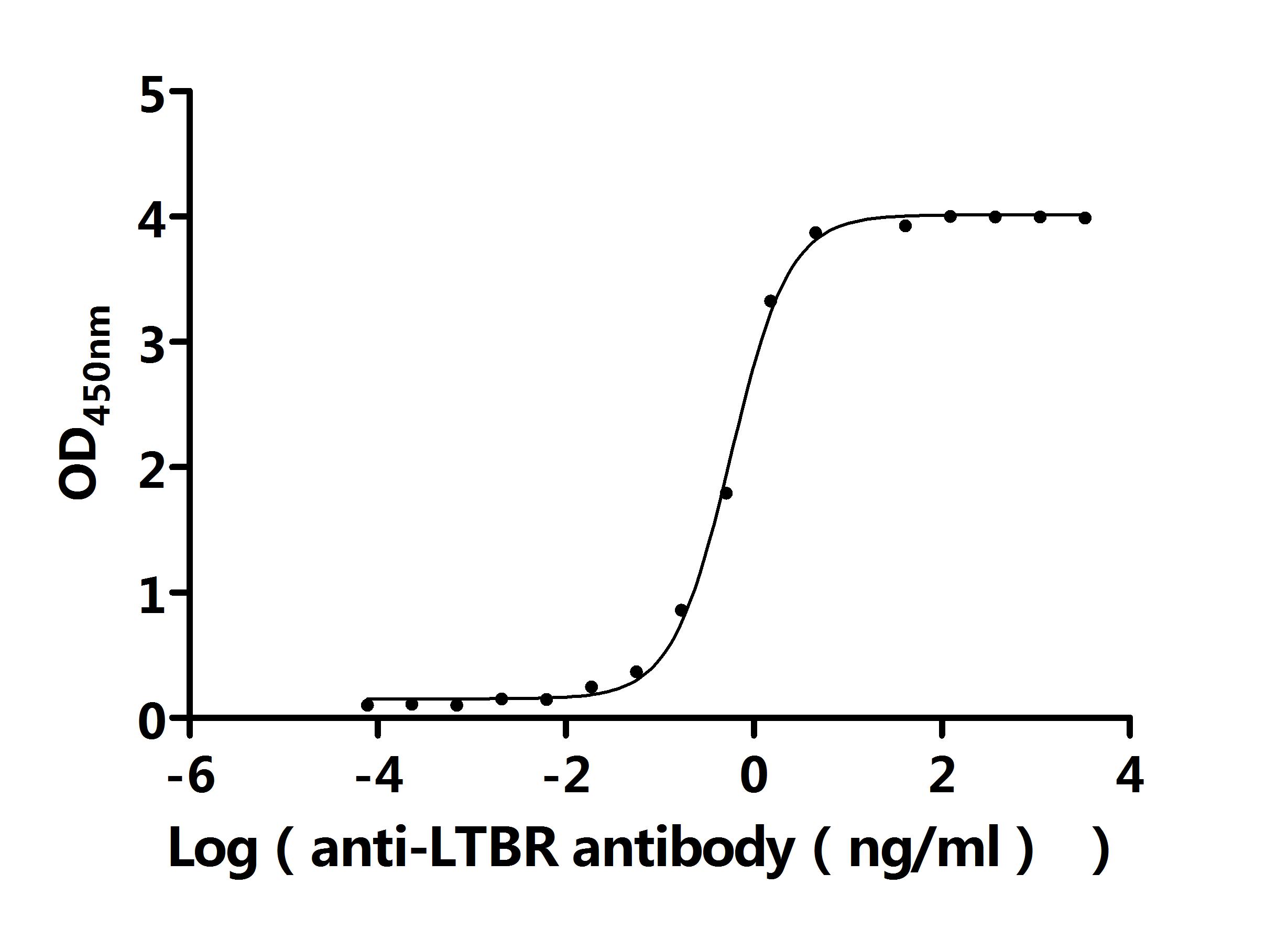
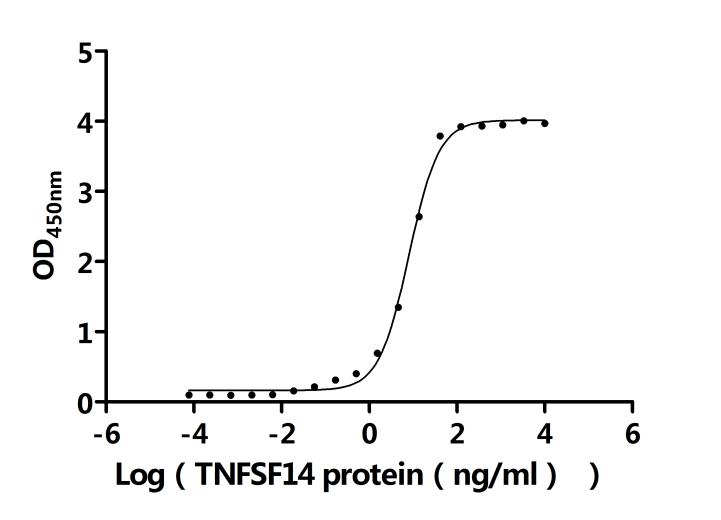
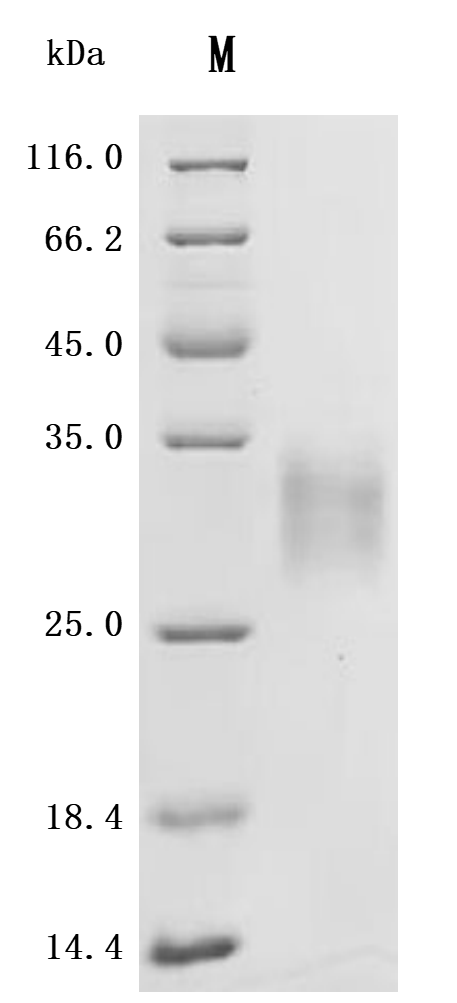
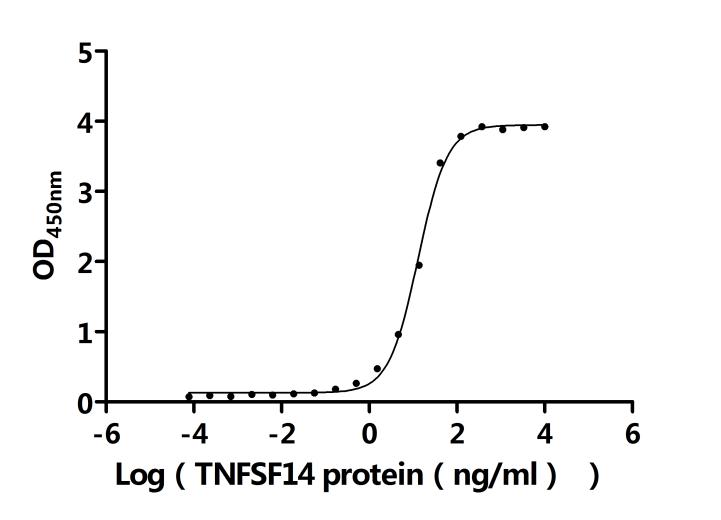
7. Recommended Products for LTBR Research
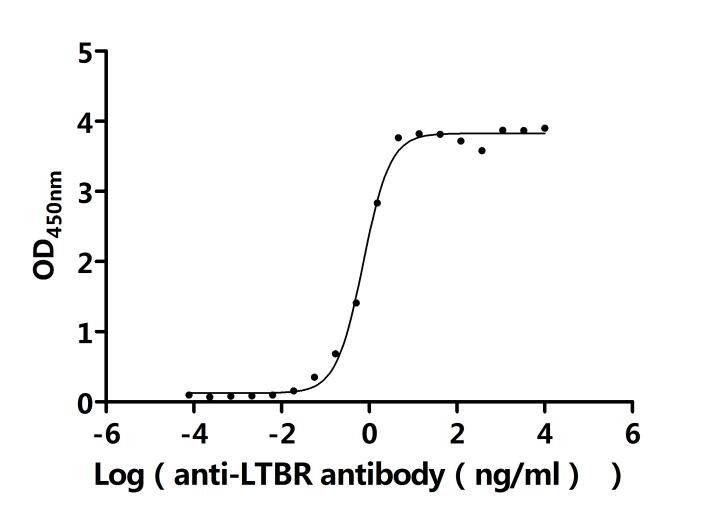
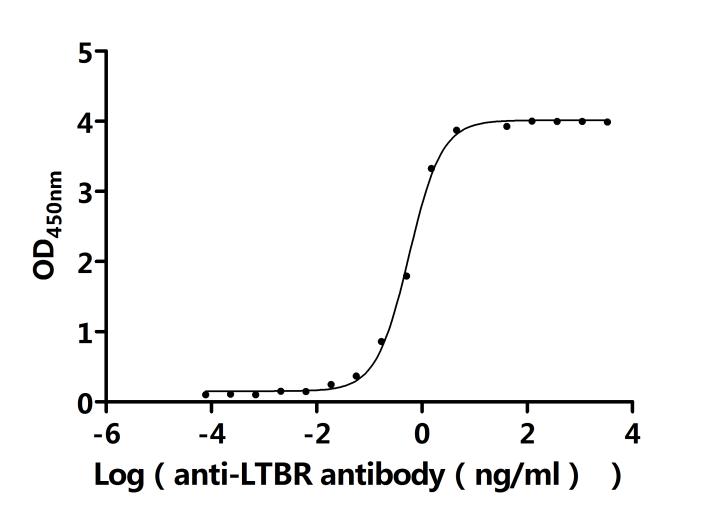
LTBR plays a crucial role in cell-to-cell communication, immune response regulation, and disease development. To assist researchers and pharmaceutical companies in studying LTBR, CUSABIO has launched a variety of high-activity LTBR protein products covering humans, mice, monkeys, and other species, as well as LTBR antibodies and ELISA kits to aid in the exploration of LTBR mechanisms and its potential clinical value.
● CUSABIO LTBR Recombinant Proteins
● CUSABIO LTBR Antibodies
Click to View CUSABIO All LTBR-Related Products.
References
[1] Chen Y, et al. Crystal structure of the lymphotoxin - beta receptor extracellular domain. Structure. 2002;10 (1):109 - 120.
[2] Smith C A, et al. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76 (6):959 - 962.
[3] Browning J L, et al. Lymphotoxin beta receptor expression and signaling. The Journal of experimental medicine. 1994;180 (4):1473 - 1478.
[4] Cheng G, et al. TRAF2 and TRAF5 mediate NF - kappa B activation by the lymphotoxin beta receptor. Molecular and cellular biology. 1995;15 (12):6709 - 6717.
[5] Ware C F, et al. Lymphotoxin - beta receptor - deficient mice lack peripheral lymph nodes, splenic follicular dendritic cells, and a subset of B cells. Cell. 1996;87 (3):1185 - 1194.
[6] Mebius R E, et al. Lymphotoxin and tumor necrosis factor regulate distinct aspects of early spleen organogenesis. The Journal of experimental medicine. 1998;187 (11):1657 - 1668.
[7] Grewal I S, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity. 1999;10 (4):435 - 443.
[8] Senftleben U, et al. Activation by IKKalpha of a second, evolutionary conserved, NF - kappaB signalling pathway. Nature. 2000;406 (6798):86 - 90.
[9] Kikly K, et al. Lymphotoxin - beta receptor engagement is required for the development of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 1999;190 (7):1073 - 1080.
[10] Kerdiles Y M, et al. Inducing tertiary lymphoid structures in tumours using a LIGHT - based fusion protein. Nature. 2015;521 (7553):365 - 369.
[11] Panni R, et al. Systematic analysis of the prognostic value and immunological function of LTBR in human cancer. Aging. 2020;12 (12):11634 - 11653.
[12] Herbst R S, et al. Abstract LB123: Conditionally active, therapeutic lymphotoxin beta receptor (LTBR) agonist bispecific antibodies for induction of tertiary lymphoid structures in the treatment of solid tumors. Cancer Research. 2022;82 (4_Supplement):LB123 - LB123.
[13] Piao W, et al. LTβR Signaling Controls Lymphatic Migration of Immune Cells. Cells. 2021;10 (4):747. doi:10.3390/cells10040747.
CUSABIO team. The Structure, Function, and Research Progress of Lymphotoxin β Receptor (LTBR) in Disease and Drug Development. https://www.cusabio.com/c-21211.html





Comments
Leave a Comment