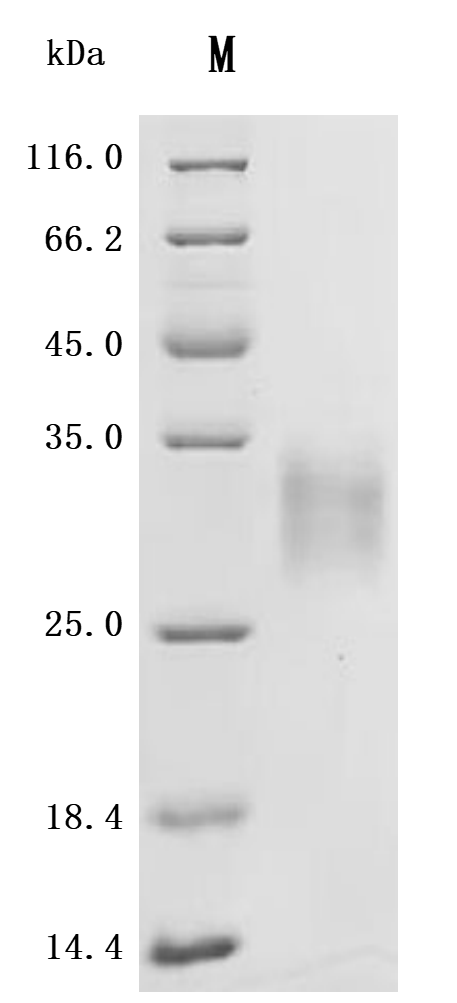
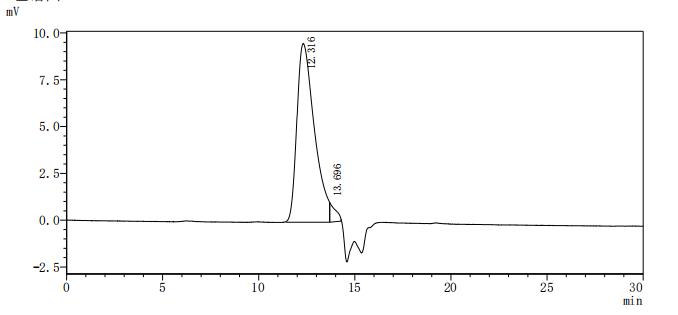
The recombinant mouse LTBR protein is a biologically active molecule generated in mammalian cells, ensuring accurate folding and post-translational modifications. It includes the amino acids 31 to 223 of mouse LTBR and carries a C-terminal 10xHis tag to support efficient purification and downstream applications. This recombinant LTBR protein is of high quality, with purity levels exceeding 95% as confirmed by both SDS-PAGE and SEC-HPLC analyses. Endotoxin levels are strictly controlled, remaining below 1.0 EU/µg through LAL testing. Functional validation in a binding ELISA demonstrates that immobilized LTBR at 2 μg/mL specifically interacts with human TNFSF14 (CSB-MP023991HUj7-B), yielding an EC50 between 15.43 and 18.78 ng/mL. These features confirm its suitability for studies focused on lymphotoxin signaling, receptor-ligand interactions, and immunological research.
The LTBR plays crucial roles in various biological processes related to immune responses, lymphoid organ development, and maintenance of structural integrity in secondary lymphoid tissues. In mice, LTBR signaling is essential for the homeostatic function of high endothelial venules (HEVs), which are critical for lymphocyte trafficking and lymph node (LN) maturity. LTBR signaling influences the differentiation and function of endothelial cells, which are integral to forming LNs and maintaining their architecture [1][2].
Research demonstrates that LTBR is necessary for maintaining follicular reticular networks, which protect the structural integrity of LNs and the splenic marginal zone [3][4]. The expression of LTBR on stromal cells also plays a critical role in the organogenesis of LNs, as it facilitates interactions between different cell types necessary for the development and functionality of these immune structures [5]. For instance, absence or blockage of LTBR leads to significant defects in the formation and function of LNs, revealing its necessity for effective immune responses [6][7].
In addition to its role in LNs, LTBR is implicated in various inflammatory diseases. Its signaling pathway has been associated with proinflammatory roles in conditions such as rheumatoid arthritis and other autoimmune diseases [3][8]. Furthermore, LTBR contributes to the host response against intracellular pathogens; mice deficient in LTBR show increased susceptibility to infections from pathogens like Mycobacterium tuberculosis [6][7]. This indicates the receptor's involvement not only in lymphoid architecture but also in immune surveillance.
Moreover, studies have highlighted the interaction of LTBR with T-cell subsets, affecting their effector functions and maturation processes. LTBR signaling is known to enhance the effector capabilities of T cells through its ligand LTB, thereby playing a critical role in T-cell biology, particularly in modulating responses during autoimmunity and infections [9][10].
References:
[1] T. Bekkhus, T. Martikainen, et al. Remodeling of the lymph node high endothelial venules reflects tumor invasiveness in breast cancer and is associated with dysregulation of perivascular stromal cells. Cancers, vol. 13, no. 2, p. 211, 2021. https://doi.org/10.3390/cancers13020211
[2] L. Onder, R. Danuser, et al. Endothelial cell–specific lymphotoxin-β receptor signaling is critical for lymph node and high endothelial venule formation. The Journal of Experimental Medicine, vol. 210, no. 3, p. 465-473, 2013. https://doi.org/10.1084/jem.20121462
[3] J. Browning, N. Allaire, et al. Lymphotoxin-β receptor signaling is required for the homeostatic control of hev differentiation and function. Immunity, vol. 23, no. 5, p. 539-550, 2005. https://doi.org/10.1016/j.immuni.2005.10.002
[4] M. Grandoch, K. Feldmann, et al. Deficiency in lymphotoxin β receptor protects from atherosclerosis in apoe-deficient mice. Circulation Research, vol. 116, no. 8, 2015. https://doi.org/10.1161/circresaha.116.305723
[5] Z. Wang, Q. Chai, & M. Zhu/ Differential roles of ltβr in endothelial cell subsets for lymph node organogenesis and maturation. The Journal of Immunology, vol. 201, no. 1, p. 69-76, 2018. https://doi.org/10.4049/jimmunol.1701080
[6] S. Ehlers, C. Hölscher, et al. The lymphotoxin β receptor is critically involved in controlling infections with the intracellular pathogens mycobacterium tuberculosis and listeria monocytogenes. The Journal of Immunology, vol. 170, no. 10, p. 5210-5218, 2003. https://doi.org/10.4049/jimmunol.170.10.5210
[7] L. Onder, U. Mörbe, et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity, vol. 47, no. 1, p. 80-92.e4, 2017. https://doi.org/10.1016/j.immuni.2017.05.008
[8] K. Behnke, U. Sorg, D. Herebıan, D. Häussinger, V. Keitel, & K. Pfeffer. The role of the lymphotoxin-β receptor (ltβr) in hepatocyte-mediated liver regeneration. European Journal of Medical Research, vol. 19, no. S1, 2014. https://doi.org/10.1186/2047-783x-19-s1-s3
[9] J. Gommerman, K. Giza, et al. A role for surface lymphotoxin in experimental autoimmune encephalomyelitis independent of light. Journal of Clinical Investigation, vol. 112, no. 5, p. 755-767, 2003. https://doi.org/10.1172/jci18648
[10] L. Xia and H. Ma. Identification of a novel signature based on ferritinophagy-related genes to predict prognosis in lung adenocarcinoma: focus on ahnak2. Bioengineering, vol. 11, no. 11, p. 1070, 2024. https://doi.org/10.3390/bioengineering11111070